Successive amino acids in proteins are connected by peptide bonds.
Amino acids are linked together to form proteins. Fig. 4.3 shows how amino acids in a protein are bonded together. The bond formed between the two amino acids is a peptide bond, shown in red in Fig. 4.3. In forming the peptide bond, the carboxyl group of one amino acid reacts with the amino group of the next amino acid in line, and a molecule of water is released. Note that, in the resulting molecule, the R groups of each amino acid point in different directions.
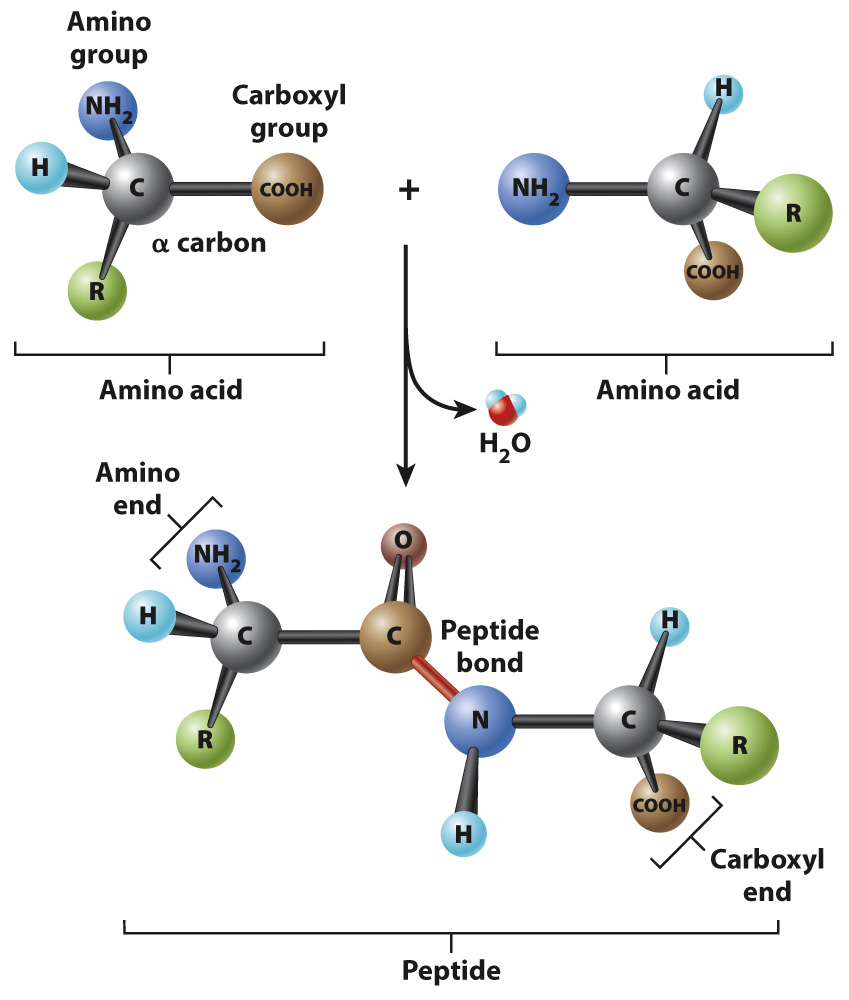
The C=O group in the peptide bond is known as a carbonyl group, and the N–
Polymers of amino acids ranging from as few as two to many hundreds share a chemical feature common to individual amino acids: namely, that the ends are chemically distinct from each other. One end, shown at the left in Fig. 4.3, has a free amino group; this is the amino end of the molecule. The other end has a free carboxyl group, which is the carboxyl end of the molecule. More generally, a polymer of amino acids connected by peptide bonds is known as a polypeptide. Typical polypeptides produced in cells consist of a few hundred amino acids. In human cells, the shortest polypeptides are about 100 amino acids in length; the longest is the muscle protein titin, with 34,350 amino acids. The term protein is often used as a synonym for polypeptide, especially when the polypeptide chain has folded into a stable, three-![]() and COO–, respectively. However, for simplicity, we denote the ends as NH2 and COOH.
and COO–, respectively. However, for simplicity, we denote the ends as NH2 and COOH.
73