The sequence of amino acids dictates protein folding, which determines function.
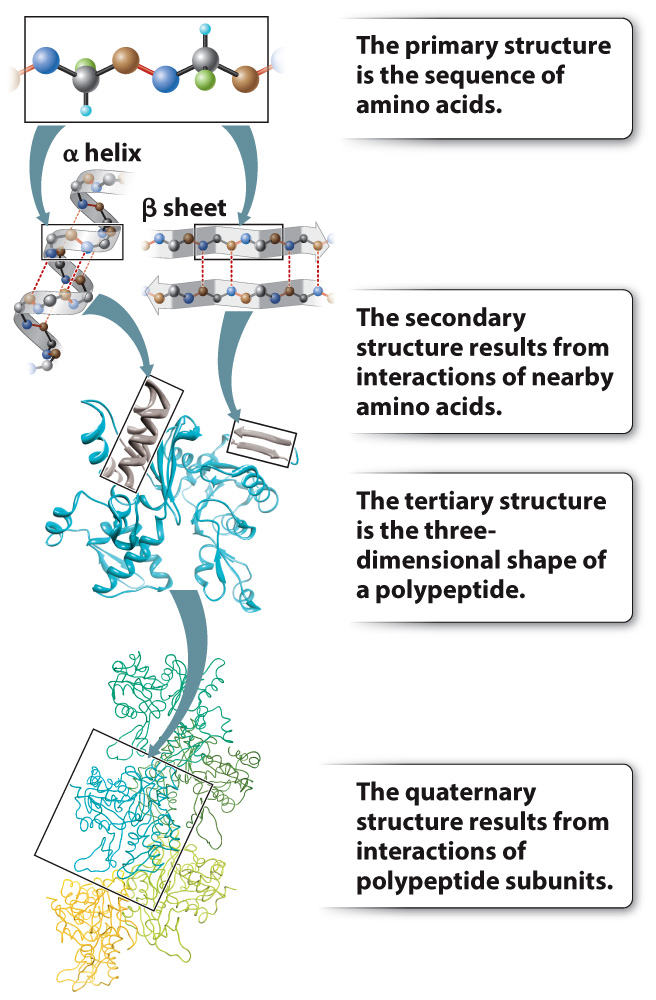
FIG. 4.4 Levels of protein structure. The manner in which a polypeptide folds determines its shape and function.
Up to this point, we have considered the sequence of amino acids that make up a protein. This is the first of several levels of protein structure, illustrated in Fig. 4.4. The sequence of amino acids in a protein is its primary structure. The sequence of amino acids ultimately determines how a protein folds. Interactions between stretches of amino acids in a protein form local secondary structures. Longer-range interactions between these secondary structures in turn support the overall three-dimensional shape of the polypeptide, which is its tertiary structure. Finally, some proteins are made up of several individual polypeptides that interact with each other, and the resulting ensemble is the quaternary structure.
Proteins have a remarkably wide range of functions in the cell, from serving as structural elements to communicating with the external environment to accelerating the rate of chemical reactions. No matter what the function of a protein is, the ability to carry out this function depends on the three-dimensional shape of the protein. When fully folded, some proteins contain pockets with positively or negatively charged side chains at just the right positions to trap small molecules; others have surfaces that can bind another protein or a sequence of nucleotides in DNA or RNA; some form rigid rods for structural support; and still others keep their hydrophobic side chains away from water molecules by inserting into the cell membrane.
The sequence of amino acids in a protein (its primary structure) is usually represented by a series of three-letter or one-letter abbreviations for the amino acids (abbreviations for the 20 common amino acids are given in Fig. 4.2). By convention, the amino acids in a protein are listed in order from left to right, starting at the amino end and proceeding to the carboxyl end. The amino and the carboxyl ends are different, so the order matters. Just as TIPS is not the same word as SPIT, the sequence Thr–Ile–Pro–Ser is not the same polypeptide as Ser–Pro–Ile–Thr.
