Tertiary structures result from interactions between amino acid side chains.
The tertiary structure of a protein is the three-dimensional conformation of a single polypeptide chain, usually made up of several secondary structure elements. The shape of a protein is defined largely by interactions between the amino acid R groups. By contrast, the formation of secondary structures relies on interactions in the polypeptide backbone and is relatively independent of the R groups. Tertiary structure is determined by the spatial distribution of hydrophilic and hydrophobic R groups along the molecule, as well as by different types of chemical bonds and interactions (ionic, hydrogen, and van der Waals) that form between various R groups. The amino acids whose R groups form bonds with each other may be far apart in the polypeptide chain, but can end up near each other in the folded protein. Hence, the tertiary structure usually includes loops or turns in the backbone that allow these R groups to sit near each other in space and for bonds to form.
The three-dimensional shapes of proteins can be illustrated in different ways, as shown in Fig. 4.8. A ball-and-stick model (Fig. 4.8a) draws attention to the atoms in the amino acid chain. A ribbon model (Fig. 4.8b) emphasizes secondary structures, with α helices depicted as twisted ribbons and β sheets as broad arrows. Finally, a space-filling model (Fig. 4.8c) shows the overall shape and contour of the folded protein.
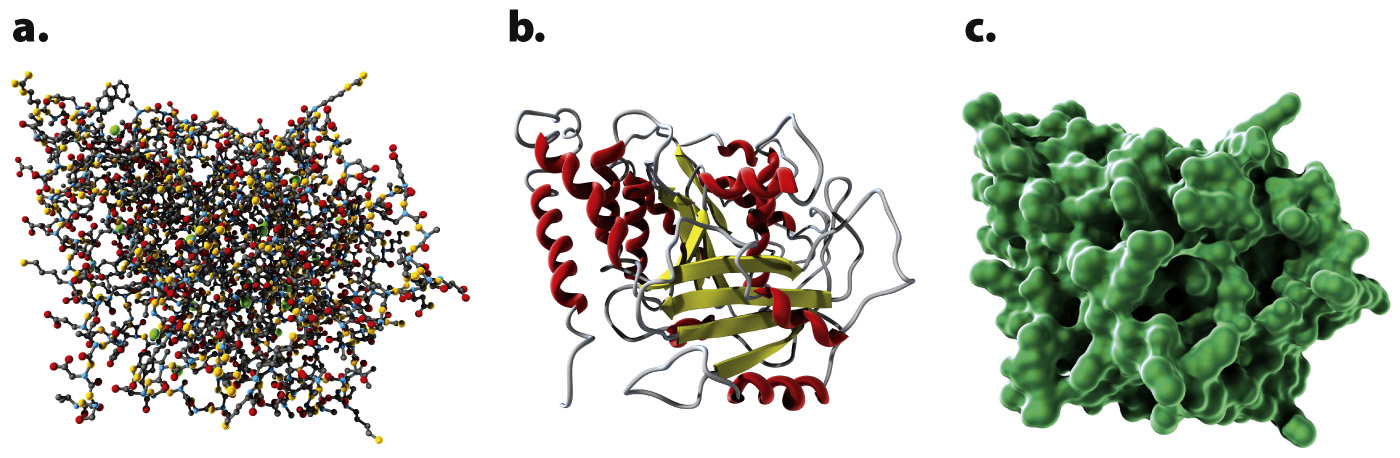
FIG. 4.8 Three ways of showing the structure of the protein tubulin: (a) ball-and-stick model; (b) ribbon model; (c) space-filling model.
Remember that the folding of a polypeptide chain is determined by the sequence of amino acids. The primary structure determines the secondary and tertiary structures. Furthermore, tertiary structure determines function because it is the three-dimensional shape of the molecule—the contours and distribution of charges on the outside of the molecule and the presence of pockets that might bind with smaller molecules on the inside—that enables the protein to serve as structural support, membrane channel, enzyme, or signaling molecule. Fig. 4.9 shows the tertiary structure of a bacterial protein that contains a pocket in the center in which certain R groups can form hydrogen bonds with a specific small molecule and hold it in place.
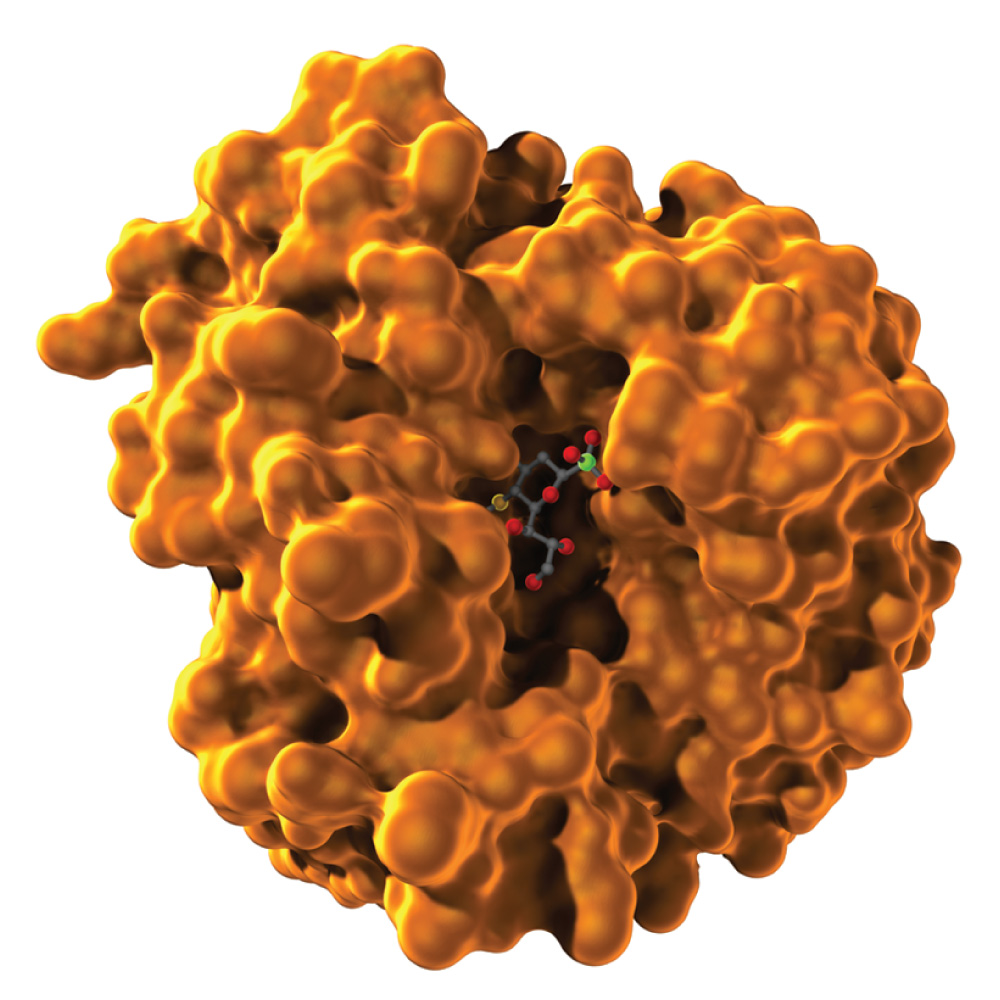
FIG. 4.9 Tertiary structure determines function. This bacterial protein has a cavity that can bind with a small molecule (shown as a ball-and-stick model in the center).
The principle that structure determines function can be demonstrated by many observations. For example, most proteins can be unfolded, or denatured, by chemical treatment or high temperature that disrupts the hydrogen and ionic bonds holding the tertiary structure together. Under these conditions, the proteins lose their functional activity. Similarly, mutant proteins containing an amino acid that prevents proper folding are often inactive or don’t function properly.
Quick Check 1 A mutation leads to a change in one amino acid in a protein. The result is that the protein no longer functions properly. How is this possible?
Quick Check 1 Answer
The sequence of amino acids in a protein determines how a protein folds, so a change in even a single amino acid can affect the way the protein folds and can disrupt its function. For example, if the hydrophobic R groups of two amino acids must aggregate for proper structure and function, then a mutation that changes one of the hydrophobic amino acids for an acidic or a basic amino acid will prevent this aggregation and disrupt structure and function. Similarly, if proper folding requires interaction between the R groups of an acidic and a basic amino acid, then, if either one of them is changed to a hydrophobic amino acid, proper folding will not take place.

