Secondary active transport is driven by an electrochemical gradient.
Active transport can also work in another way. Because small ions cannot cross the lipid bilayer, many cells use a transport protein to build up the concentration of a small ion on one side of the membrane. The resulting concentration gradient stores potential energy that can be harnessed to drive the movement of other substances across the membrane against their concentration gradient.
For example, some cells actively pump protons (H+) across the cell membrane using ATP (Fig. 5.13a). As a result, in these cells the concentration of protons is higher on one side of the membrane and lower on the other side. In other words, the pump generates a concentration gradient, also called a chemical gradient because the entity forming the gradient is a chemical (Fig. 5.13b). We have already seen that concentration differences favor the movement of protons back to the other side of the membrane. However, the lipid bilayer blocks the movement of protons to the other side and therefore stores potential energy, just like a dam or battery.
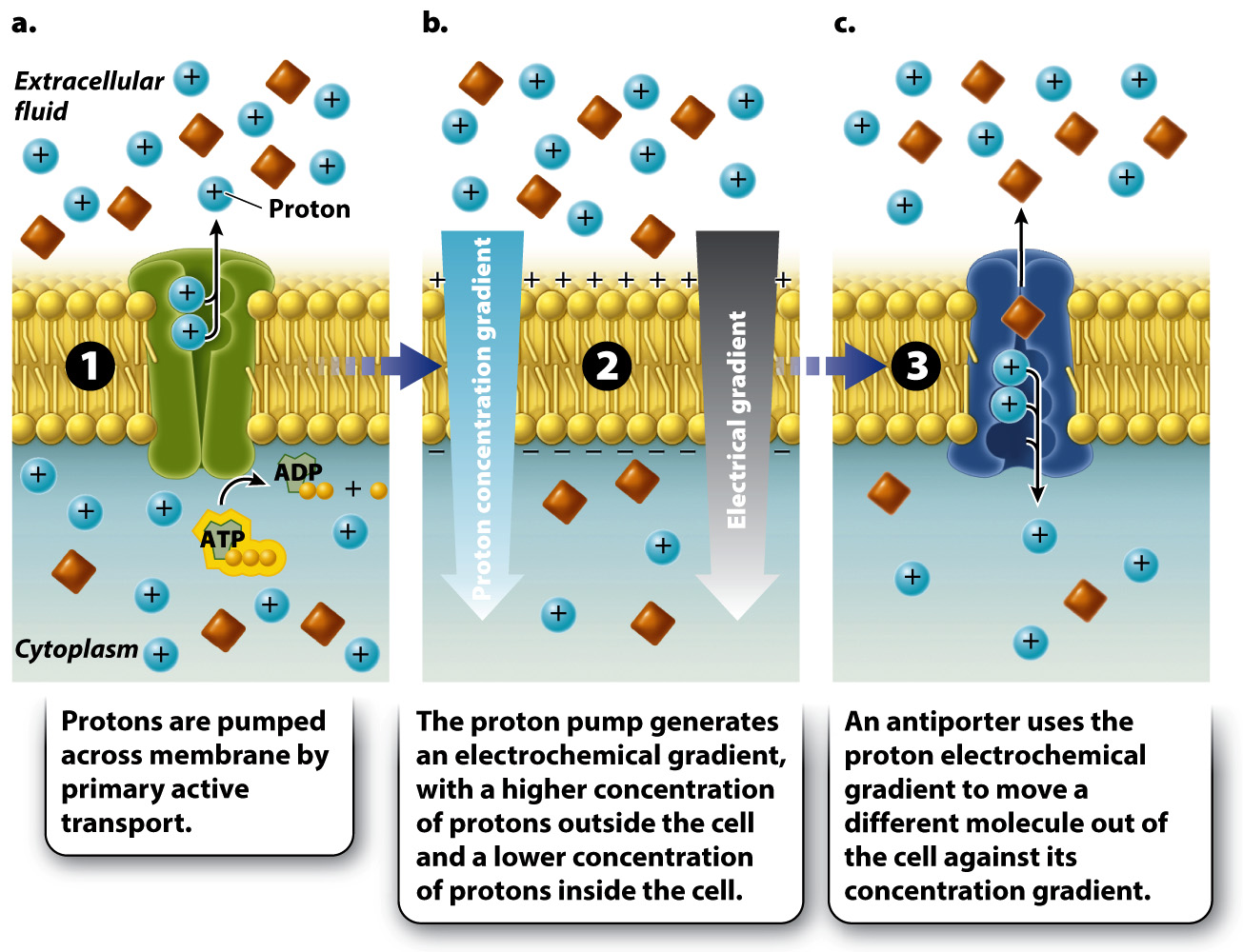
In addition to the chemical gradient, another force favors the movement of protons back across the membrane: a difference in charge. Because protons carry a positive charge, the side of the membrane with more protons is more positive than the other side. This difference in charge is called an electrical gradient. Protons (and other ions) move from areas of like charge to areas of unlike charge, driven by an electrical gradient. A gradient that has both charge and chemical components is known as an electrochemical gradient (Fig. 5.13b).
99
If protons are then allowed to pass through the cell membrane by a transport protein, they will move down their electrochemical gradient toward the region of lower proton concentration. These transport proteins can use the movement of protons to drive the movement of other molecules against their concentration gradient (Fig. 5.13c). The movement of protons is always from regions of higher to lower concentration, whereas the movement of the coupled molecule is from regions of lower to higher concentration. Because the movement of the coupled molecule is driven by the movement of protons and not by ATP directly, this form of transport is called secondary active transport. Secondary active transport uses the potential energy of an electrochemical gradient to drive the movement of molecules; by contrast, primary active transport uses the chemical energy of ATP directly.
The use of an electrochemical gradient as a temporary energy source is a common cellular strategy. For example, cells use a sodium electrochemical gradient generated by the sodium-