Cell membranes are composed of two layers of lipids.
The major types of lipid found in cell membranes are phospholipids, introduced in Chapter 2. Most phospholipids are made up of a glycerol backbone attached to a phosphate group and two fatty acids (Fig. 5.2). The phosphate head group is hydrophilic (“water-loving”) because it is polar, enabling it to form hydrogen bonds with water. By contrast, the two fatty acid tails are hydrophobic (“water-fearing”) because they are nonpolar and do not form hydrogen bonds with water. Molecules with both hydrophilic and hydrophobic regions in a single molecule are termed amphipathic.
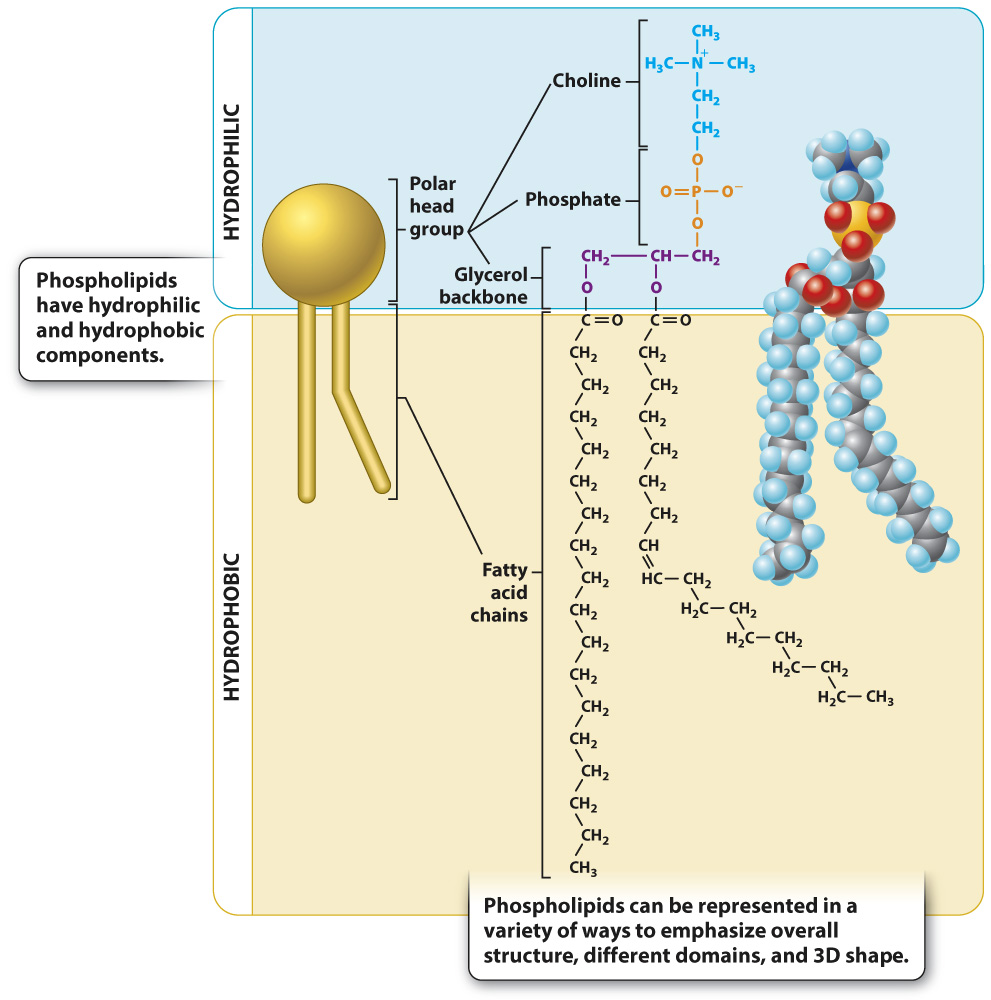
FIG. 5.2 Phospholipid structure. Phospholipids, the major component of cell membranes, are made up of glycerol attached to a phosphate-containing head group and two fatty acid tails. They are amphipathic because they have both hydrophilic and hydrophobic domains.
In an aqueous environment, amphipathic molecules such as phospholipids behave in an interesting way. They spontaneously arrange themselves into various structures in which the polar head groups on the outside interact with water and the nonpolar tail groups come together on the inside away from water. This arrangement results from the tendency of polar molecules like water to exclude nonpolar molecules or nonpolar groups of molecules.
The shape of the structure is determined by the bulkiness of the head group relative to the hydrophobic tails. For example, lipids with bulky heads and a single hydrophobic fatty acid tail are wedge-shaped and pack into spherical structures called micelles (Fig. 5.3a). By contrast, lipids with less bulky head groups and two hydrophobic tails form a bilayer (Fig. 5.3b). A lipid bilayer is a structure formed of two layers of lipids in which the hydrophilic heads are the outside surfaces of the bilayer and the hydrophobic tails are sandwiched in between, isolated from contact with the aqueous environment.
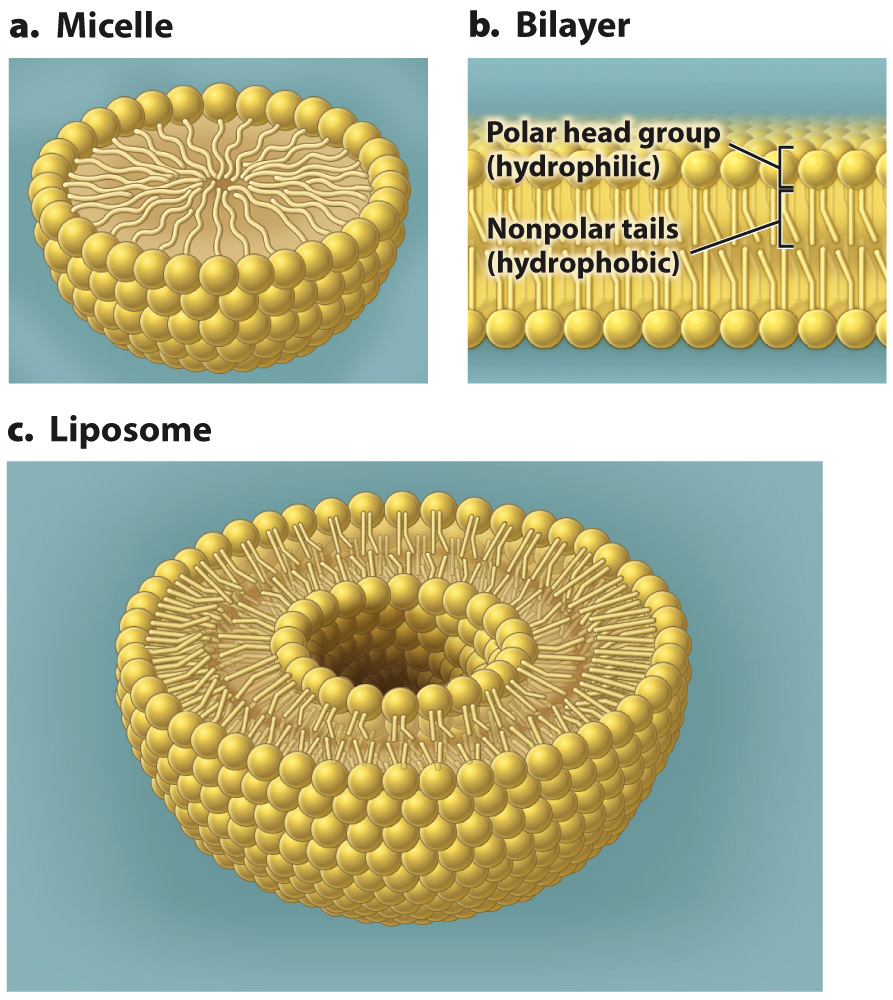
FIG. 5.3 Phospholipid structures. Phospholipids can form (a) micelles, (b) bilayers, or (c) liposomes when placed in water.
The bilayers form closed structures with an inner space since free edges would expose the hydrophobic chains to the aqueous environment. This organization in part explains why bilayers are effective cell membranes. It also explains why membranes are self-healing. Small tears in a membrane are rapidly sealed by the spontaneous rearrangement of the lipids surrounding the damaged region because of the tendency of water to exclude nonpolar molecules.

