Cell membranes are dynamic.
Lipids freely associate with one another because of extensive van der Waals forces between their fatty acid tails (Chapter 2). These weak interactions are easily broken and re-
Because membrane lipids are able to move in the plane of the membrane, the membrane is said to be fluid. The degree of membrane fluidity depends on which types of lipid make up the membrane. In a single layer of the lipid bilayer, the strength of the van der Waals interactions between the lipids’ tails depends on the length of the fatty acid tails and the presence of double bonds between neighboring carbon atoms. The longer the fatty acid tails, the more surface is available to participate in van der Waals interactions. The tighter packing that results tends to reduce lipid mobility. Likewise, saturated fatty acid tails, which have no double bonds, are straight and tightly packed—
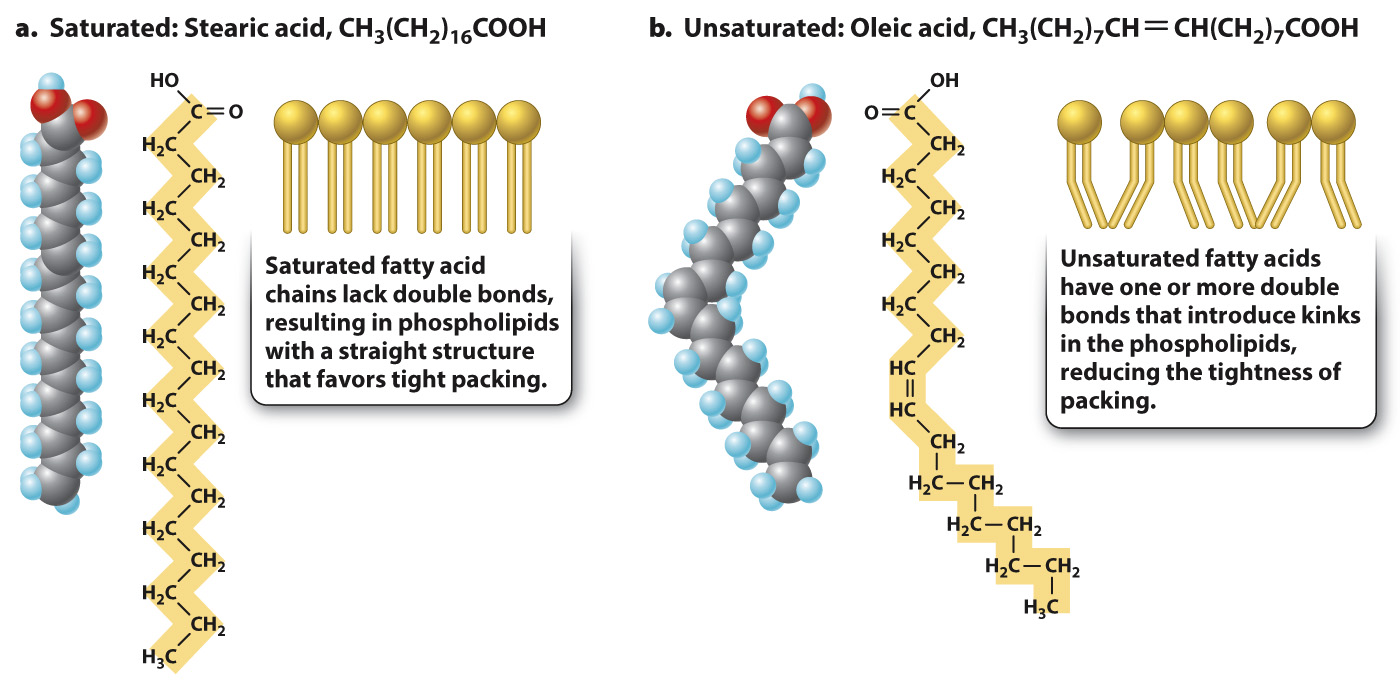
Quick Check 1 Most animal fats are solid at room temperature, whereas plant and fish oils tend to be liquid. Both contain fatty acids. Can you predict which type of fat contains saturated fatty acids, and which type contains unsaturated fatty acids?
Quick Check 1 Answer
Saturated fatty acids are less mobile within the membrane compared to unsaturated fatty acids. As a result, saturated fatty acids tend to be solid at room temperature, whereas unsaturated fatty acids tend to be liquid. Margarine and many other animal fats contain saturated fatty acids and are solid, whereas many plant and fish oils contain unsaturated fatty acids and are liquid at room temperature.
In addition to phospholipids, cell membranes often contain other types of lipid, and these can also influence membrane fluidity. For example, cholesterol is a major component of animal cell membranes, representing about 30% by mass of the membrane lipids. Like phospholipids, cholesterol is amphipathic, with both hydrophilic and hydrophobic groups in the same molecule. In cholesterol, the hydrophilic region is simply a hydroxyl group (–OH) and the hydrophobic region consists of four interconnected carbon rings with an attached hydrocarbon chain (Fig. 5.5). This structure allows cholesterol to insert into the lipid bilayer so that its head group interacts with the hydrophilic head group of phospholipids, while the ring structure participates in van der Waals interactions with the fatty acid chains.
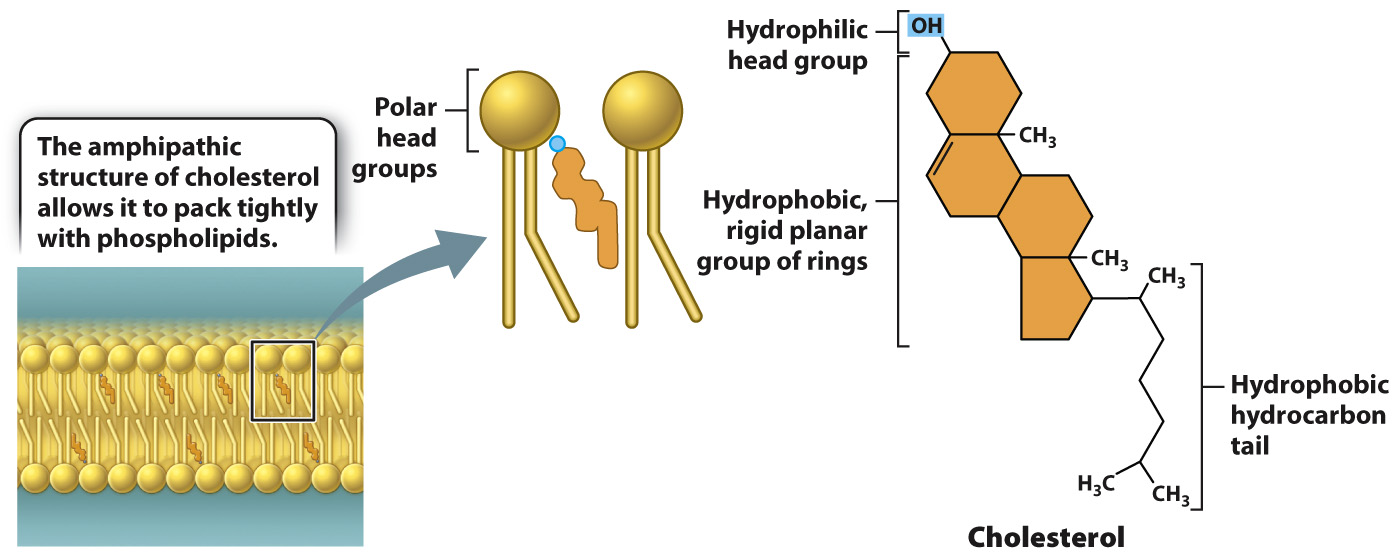
Cholesterol increases or decreases membrane fluidity depending on temperature. At temperatures typically found in a cell, cholesterol decreases membrane fluidity because the interaction of the rigid ring structure of cholesterol with the phospholipid fatty acid tails reduces the mobility of the phospholipids. However, at low temperatures, cholesterol increases membrane fluidity because it prevents phospholipids from packing tightly with other phospholipids. Thus, cholesterol helps maintain a consistent state of membrane fluidity by preventing dramatic transitions from a fluid to solid state.
93
For many decades, it was thought that the various types of lipid found in the membrane were randomly distributed throughout the bilayer. However, more recent studies show that specific types of lipid, such as sphingolipids, sometimes assemble into defined patches called lipid rafts. Cholesterol and other membrane components such as proteins also appear to accumulate in some of these regions. Thus, membranes are not always a uniform fluid bilayer, but instead can contain regions with discrete components.
Although lipids are free to move in the plane of the membrane, the spontaneous transfer of a lipid between layers of the bilayer, known as lipid flip-