Proteins associate with cell membranes in different ways.
Most membranes contain proteins as well as lipids. For example, proteins represent as much as 50% by mass of the membrane of a red blood cell. Membrane proteins serve different functions (Fig. 5.6). Some act as transporters, moving ions or other molecules across the membrane. Other membrane proteins act as receptors that allow the cell to receive signals from the environment. Still others are enzymes that catalyze chemical reactions or anchors that attach to other proteins and help to maintain cell structure and shape.
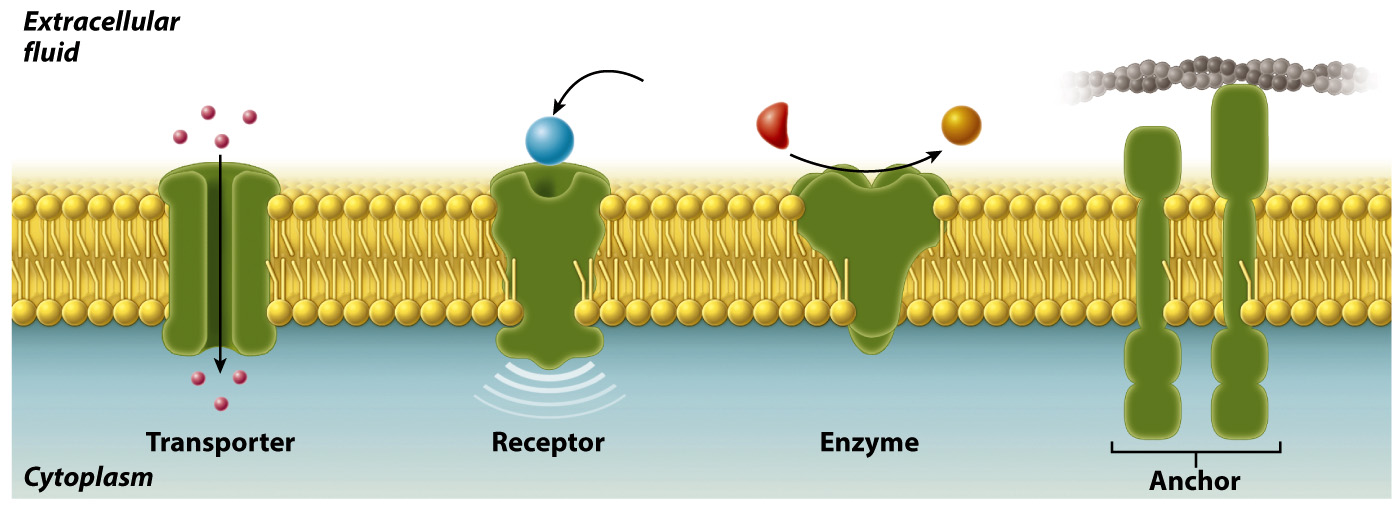
94
These various membrane proteins can be classified into two groups depending on how they associate with the membrane (Fig. 5.7). Integral membrane proteins are permanently associated with cell membranes and cannot be separated from the membrane experimentally without destroying the membrane itself. Peripheral membrane proteins are temporarily associated with the lipid bilayer or with integral membrane proteins through weak noncovalent interactions. They are easily separated from the membrane by simple experimental procedures that leave the structure of the membrane intact.
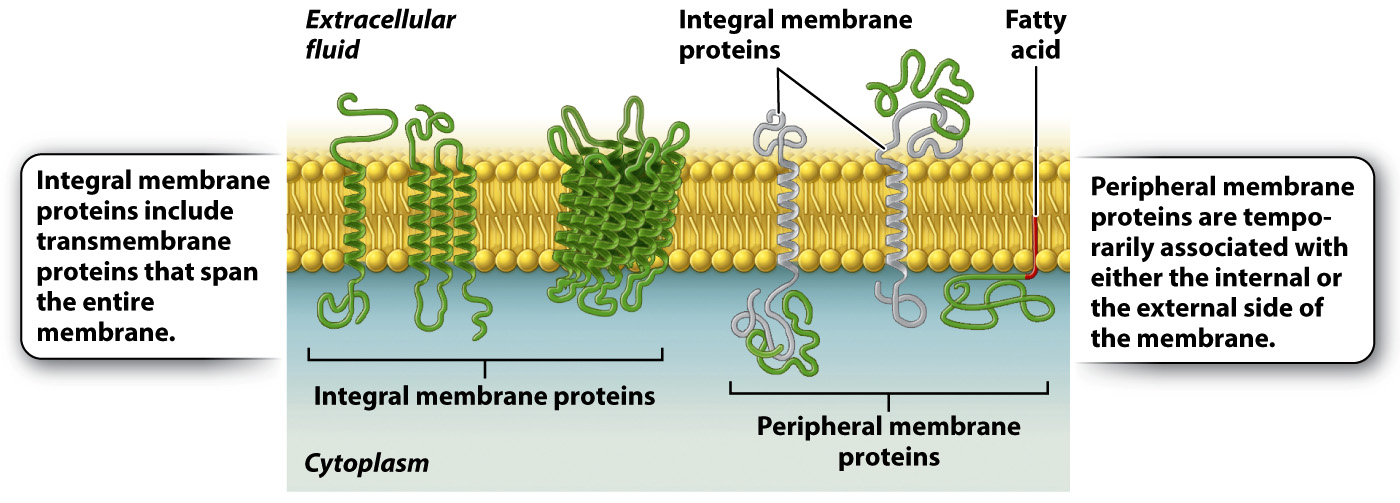
Most integral membrane proteins are transmembrane proteins that span the entire lipid bilayer, as shown in Fig. 5.7. These proteins are composed of three regions: two hydrophilic regions, one protruding from each face of the membrane, and a connecting hydrophobic region that spans the membrane. This structure allows for separate functions and capabilities of each end of the protein. For example, the hydrophilic region on the external side of a receptor can interact with signaling molecules, whereas the hydrophilic region on the internal side of the membrane often interacts with other proteins in the cytoplasm of the cell to pass along the message.
Peripheral membrane proteins may be associated with either the internal or external side of the membrane (Fig. 5.7). These proteins interact either with the polar heads of lipids or with integral membrane proteins by weak noncovalent interactions such as hydrogen bonds. Peripheral membrane proteins are only transiently associated with the membrane and can play a role in transmitting information received from external signals. Other peripheral membrane proteins limit the ability of transmembrane proteins to move within the membrane and assist proteins in clustering in lipid rafts.
Proteins, like lipids, are free to move in the membrane. How do we know this? The mobility of proteins in the cell membrane can be demonstrated using an elegant experimental technique called fluorescence recovery after photobleaching, or FRAP (Fig. 5.8). In this technique, proteins embedded in the cell membrane are labeled with fluorescent dye molecules. Labeling all the proteins in a membrane creates a fluorescent cell that can be visualized with a fluorescence microscope. A laser is then used to bleach the fluorescent dye molecules in a small area of the cell membrane, leaving a nonfluorescent spot on the surface of the cell. If proteins in the cell membrane were not capable of movement, the bleached area would remain nonfluorescent. However, over time, fluorescence appears in the bleached area, telling us that fluorescent proteins that were not bleached moved into the bleached area.
The idea that lipids and proteins coexist in the membrane, and that both are able to move in the plane of the membrane, led American biologists S. Jonathan Singer and Garth Nicolson to propose the fluid mosaic model in 1972. According to this model, the lipid bilayer is a fluid structure within which molecules move laterally, and is a mosaic (a mixture) of two types of molecules, lipids and proteins.
95
HOW DO WE KNOW?
FIG. 5.8
Do proteins move in the plane of the membrane?
BACKGROUND Fluorescent recovery after photobleaching (FRAP) is a technique used to measure mobility of molecules in the plane of the membrane. A fluorescent dye is attached to proteins embedded in the cell membrane in a process called labeling. A laser is then used to bleach a small area of the membrane.
HYPOTHESIS If membrane components such as proteins move in the plane of the membrane, the bleached spot should become fluorescent over time as unbleached fluorescent molecules move into the bleached area. If membrane components do not move, the bleached spot should remain intact.
EXPERIMENT AND RESULTS
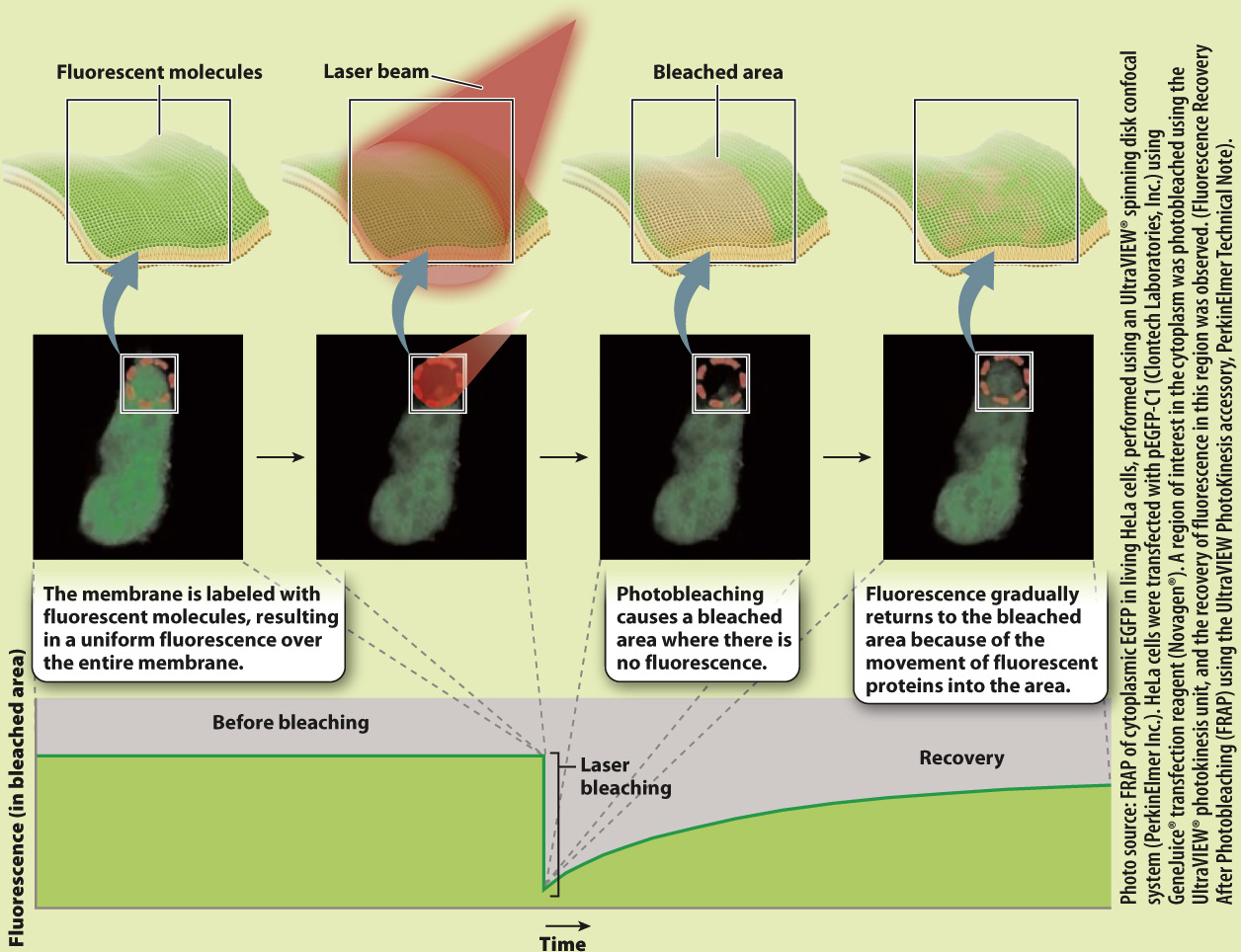
CONCLUSION The gradual recovery of fluorescence in the bleached area indicates that proteins move in the plane of the membrane.
SOURCE Peters, R., et al. 1974. “A Microfluorimetric Study of Translational Diffusion in Erythrocyte Membranes.” Biochim Biophys Acta 367:282–