Passive transport involves diffusion.
The simplest form of movement into and out of cells is passive transport. Passive transport works by diffusion, which is the random movement of molecules. Molecules are always moving in their environments. For example, molecules in water at room temperature move around at about 500 m/sec, which means that they can move only about 3 molecular diameters before they run into another molecule, leading to about 5 trillion collisions per second. The frequency with which molecules collide have important consequences for chemical reactions, which depend on the interaction of molecules (Chapter 6).
Diffusion leads to a net movement of the substance from one region to another when there is a concentration gradient in the distribution of a molecule, meaning that there are areas of higher and lower concentrations. In this case, diffusion results in net movement of the molecule from an area of higher concentration to an area of lower concentration (Fig. 5.9).
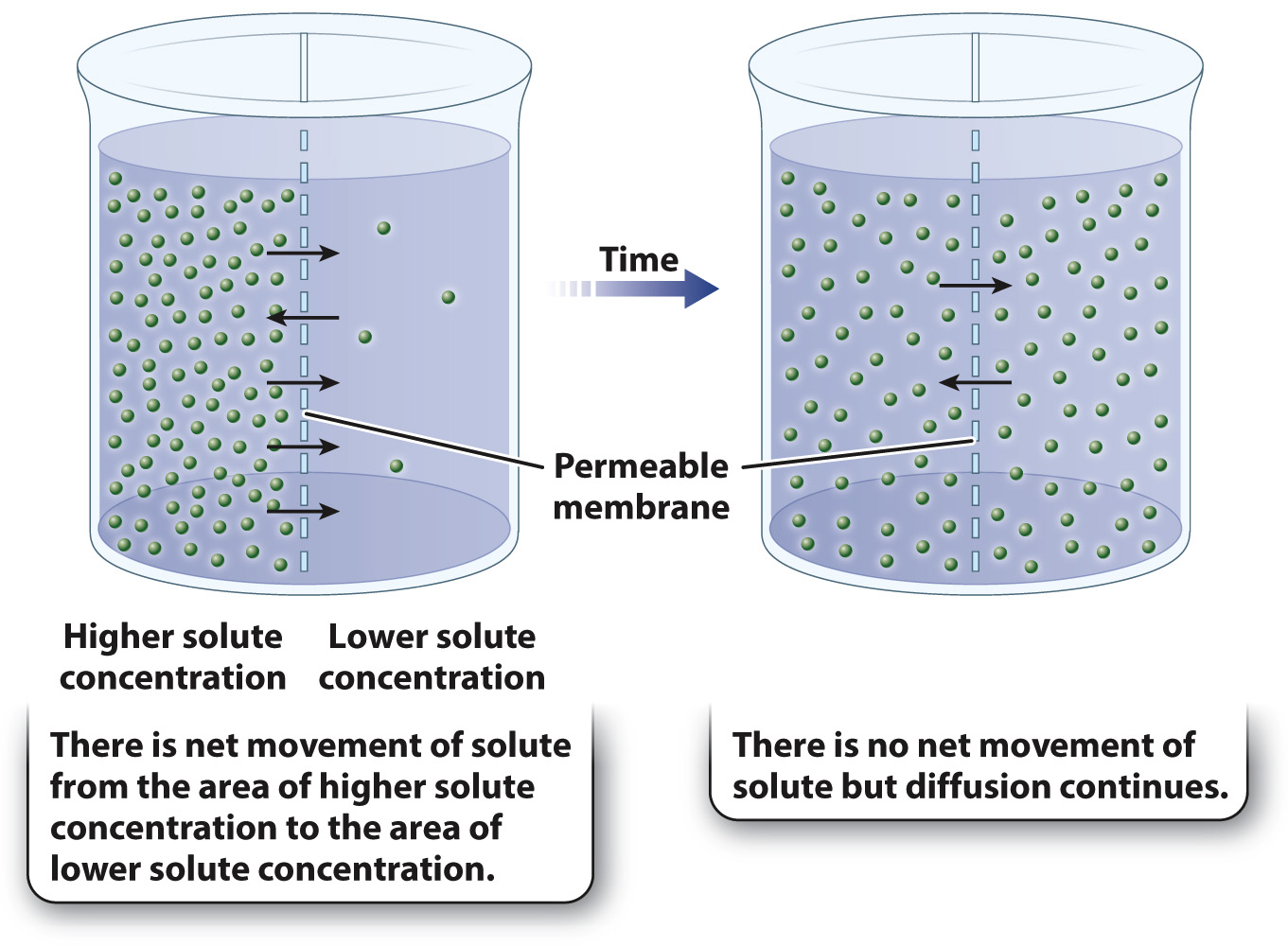
Some molecules diffuse freely across the plasma membrane as a result of differences in concentrations between the inside and the outside of a cell. Oxygen and carbon dioxide, for example, move into and out of the cell in this way. Certain hydrophobic molecules, such as triacylglycerols (Chapter 2), are also able to diffuse through the cell membrane, which is not surprising since the lipid bilayer is likewise hydrophobic.
Some molecules that cannot move across the lipid bilayer directly can move passively toward a region of lower concentration through protein transporters. When a molecule moves by diffusion through a membrane protein and bypasses the lipid bilayer, the process is called facilitated diffusion. Diffusion and facilitated diffusion both result from the random motion of molecules, and net movement of the substance occurs when there are concentration differences (Fig. 5.10). In the case of facilitated diffusion, the molecule moves through a membrane transporter, whereas in the case of simple diffusion, the molecule moves directly through the lipid bilayer.
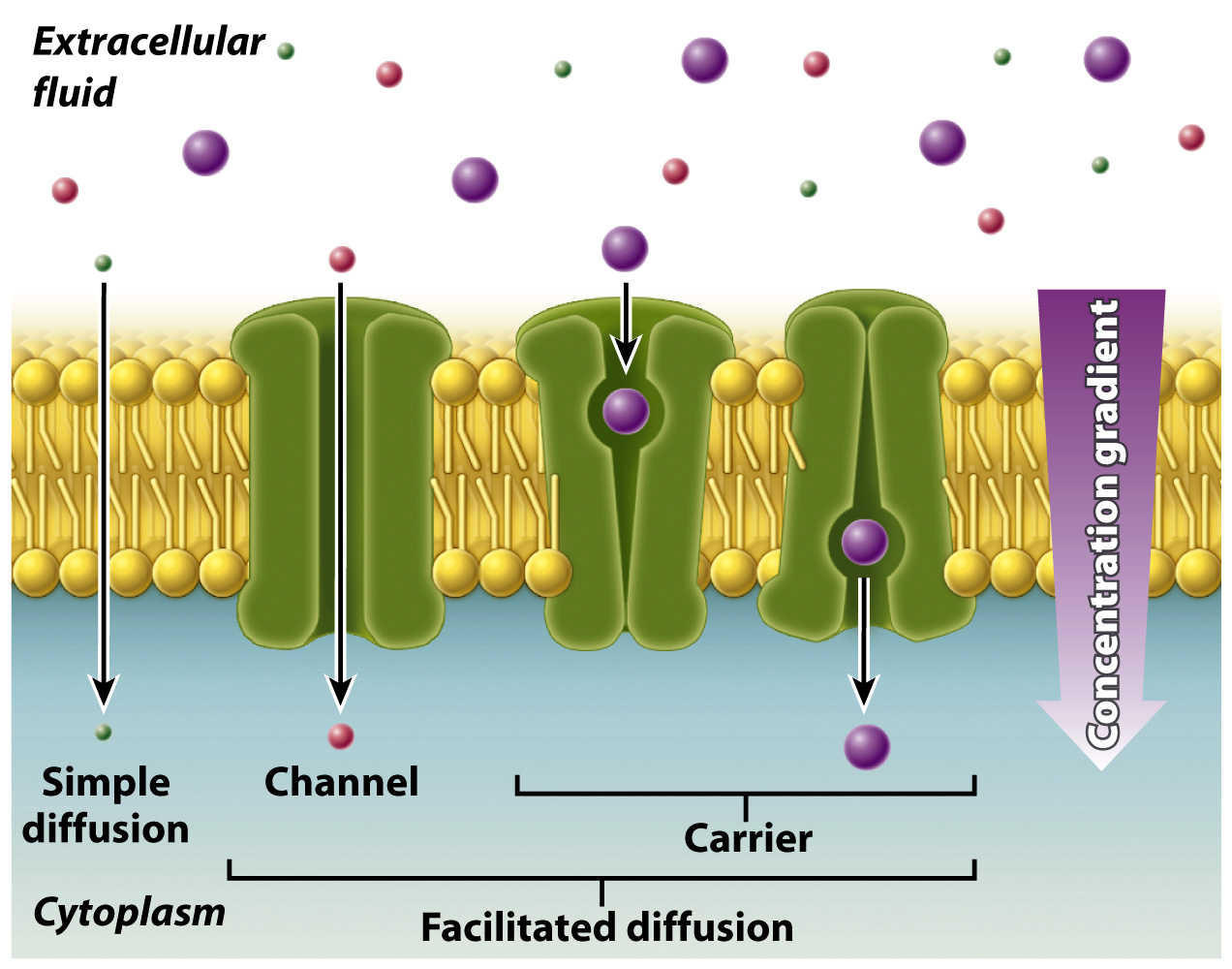
Membrane transporters are of two types. The first type is a channel, which provides an opening between the inside and outside of the cell within which certain molecules can pass, depending on their shape and charge. Some membrane channels are gated, which means that they open in response to some sort of signal, which may be chemical or electrical (Chapter 9). The second type of transporter is a carrier, which binds to and then transports specific molecules. Membrane carriers exist in two conformations, one that is open to one side of the cell, and another that is open to the other side of the cell. Binding of the transported molecule induces a conformational change in the membrane protein, allowing the molecule to be transported across the lipid bilayer, as shown on the right in Fig. 5.10.
97
Up to this point, we have focused our attention on the movement of molecules (the solutes) in water (the solvent). We can take a different perspective and focus instead on water movement. Water itself also moves into and out of cells by passive transport. Although the plasma membrane is hydrophobic, water molecules are small enough to move passively through the membrane to a limited extent by simple diffusion. In addition, many cells have specific protein channels, known as aquaporins, for transporting water molecules. These channels allow water to move more readily across the plasma membrane by facilitated diffusion than is possible by simple diffusion.
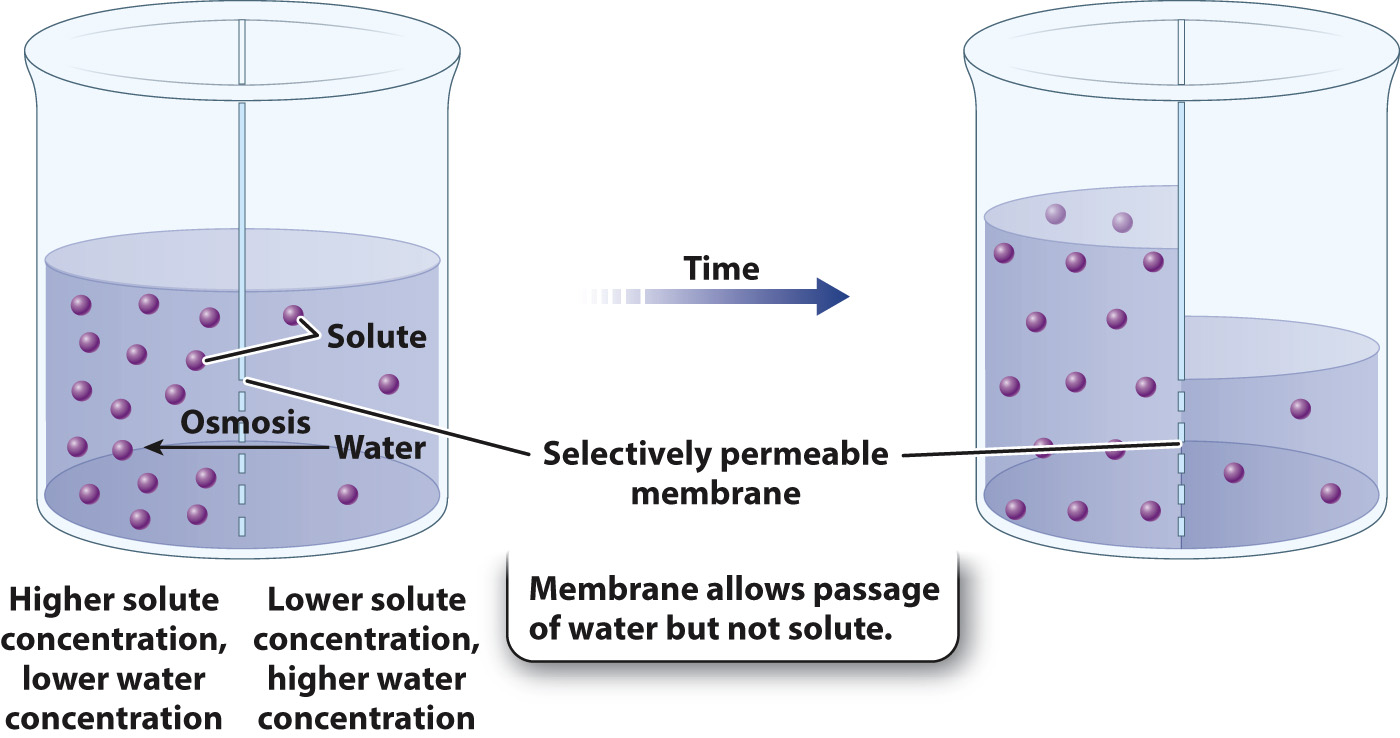
The net movement of a solvent such as water across a selectively permeable membrane such as the plasma membrane is known as osmosis. As in any form of diffusion, water moves from regions of higher water concentration to regions of lower water concentration (Fig. 5.11). Because water is a solvent within which nutrients such as glucose or ions such as sodium or potassium are dissolved, water concentration drops as solute concentration rises. Therefore, it is sometimes easier to think about water moving from regions of lower solute concentration toward regions of higher solute concentration. Either way, the direction of water movement is the same. During osmosis, the net movement of water toward the side of the membrane with higher solute concentration continues until it is opposed by another force. This force could be pressure due to gravity (in the case of Fig. 5.11) or the cell wall (in the case of plants, fungi, and bacteria, as described later in this section).
Quick Check 2 A container is divided into two compartments by a membrane that is fully permeable to water and small ions. Water is added to one side of the membrane (side A), and a 5% solution of sodium chloride (NaCl) is added to the other (side B). In which direction will water molecules move? In which direction will sodium and chloride ions move? When the concentration is equal on both sides, will diffusion stop?
Quick Check 2 Answer
Water molecules move in both directions, but the net movement of water molecules is from side A to side B. Water moves from regions of higher water concentration to regions of lower water concentration. Likewise, sodium and chloride ions move in both directions, but the net movement of sodium and chloride ions is from side B to side A. Movement of water and ions results from diffusion, the random motion of substances. Even when the concentration of all molecules is the same on the two sides, diffusion still occurs, but there is no net movement of water molecules or ions.