The first law of thermodynamics: Energy is conserved.
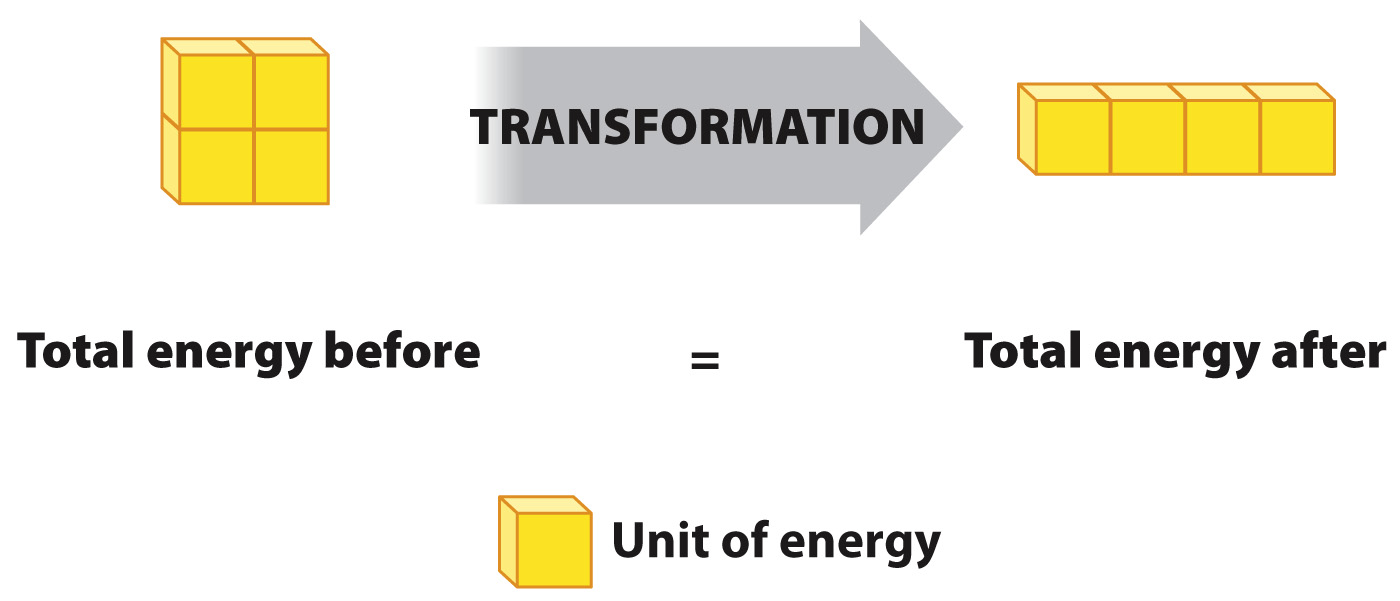
The first law of thermodynamics is the law of conservation of energy, which states that the universe contains a constant amount of energy. Therefore, energy is neither created nor destroyed. New energy is never formed, and energy is never lost. Instead, energy changes from one form to another. For example, kinetic energy can change to potential energy and vice versa, but the total amount of energy always remains the same (Fig. 6.5).
In the simple example of a ball rolling down a set of stairs (see Fig. 6.3), the potential energy stored at the top of the stairs is converted to kinetic energy associated with the movement of the ball and the surrounding air. The total amount of energy in this transformation is constant—