The second law of thermodynamics: Energy transformations always result in an increase in disorder in the universe.
When energy changes forms, the total amount of energy remains constant. However, in going from one form of energy to another, the energy available to do work decreases (Fig. 6.6). How can the total energy remain the same, but the energy available to do work decrease? The answer is that some of the energy is available to do work, and some is not available to do work. So, energy transformations are never 100% efficient since the amount of energy available to do work decreases every time energy changes forms.
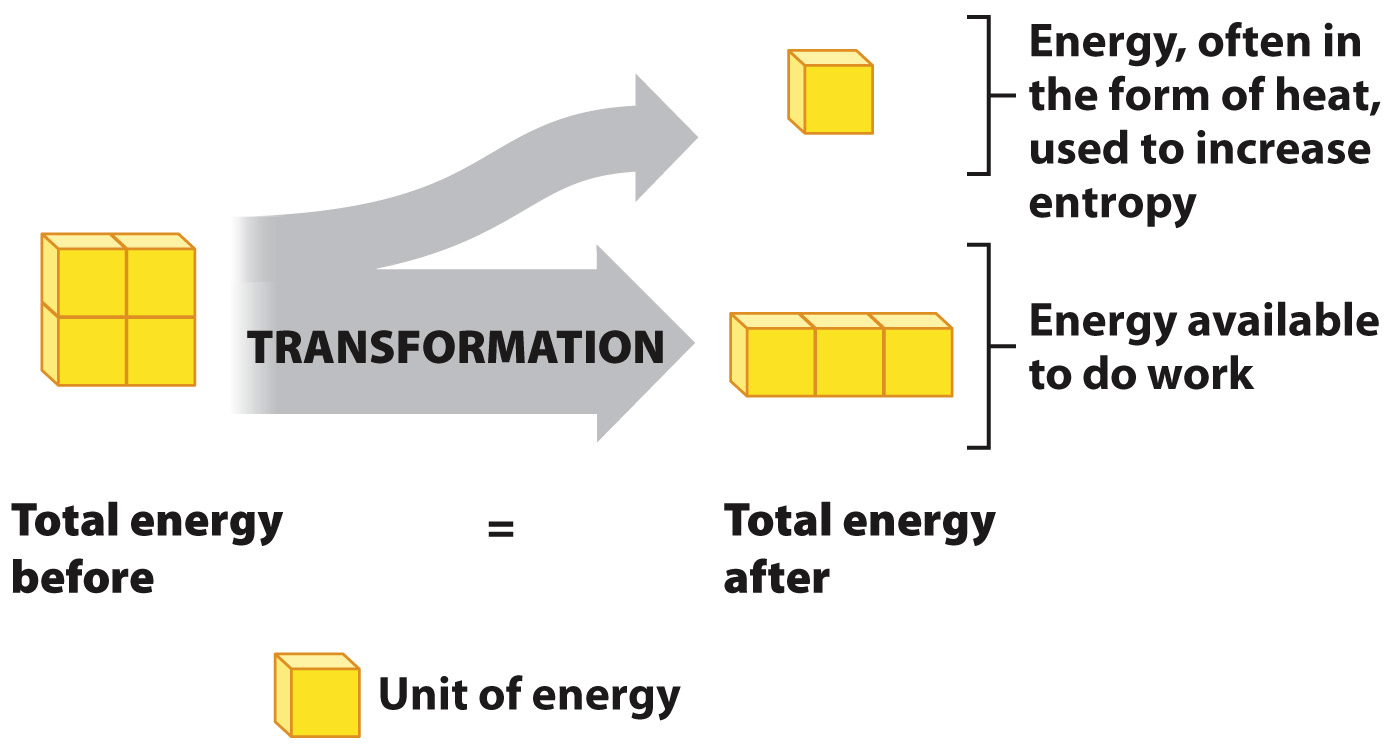
120
The energy that is not available to do work takes the form of an increase in disorder. Thus, there is a universal price to pay in transforming energy from one form to another. For example, when kinetic energy is changed into potential energy, the amount of disorder always increases. This principle is summarized by the second law of thermodynamics, which states that the transformation of energy is associated with an increase in disorder of the universe. The degree of disorder is called entropy.
The degree of disorder is just one way to describe entropy. Another way to think about entropy is to consider the number of possible positions and motions (collectively called microstates) a molecule can take on in a given system. As entropy increases, so does the number of positions and motions available to the molecule. Consider the expansion of a gas after the lid is removed from its container. Given more space, the molecules of the gas are less constrained and able to move about more freely. They have more positions available to them and move about at a larger range of speeds, so they have more entropy.
In chemical reactions, most of the entropy increase occurs through the transformation of various forms of energy into thermal energy, which we experience as heat. Thermal energy is a type of kinetic energy corresponding to the random motion of molecules and results in a given temperature. The higher the temperature, the more rapidly the molecules move, and the higher the disorder.
Consider the contraction of a muscle, a form of kinetic energy associated with the shortening of muscle cells. The contraction of muscle is powered by chemical potential energy in fuel molecules. Some of this potential energy is transformed into kinetic energy (movement), and the rest is dissipated as thermal energy (which is why your muscles warm as you exercise). The amount of chemical potential energy expended is equal to the amount of kinetic energy plus the amount of thermal energy, consistent with the first law of thermodynamics. In addition, not all of the chemical energy stored in molecules is converted to kinetic energy, as thermal energy flowing as heat is a necessary by-
In living organisms, catabolic reactions result in an increase of entropy as a single ordered biomolecule is broken down into several smaller ones with more freedom to move around. Anabolic reactions, by contrast, might seem to decrease entropy because they use individual building blocks to synthesize more ordered biomolecules such as proteins or nucleic acids. Do these anabolic reactions violate the second law of thermodynamics? No, because the second law of thermodynamics always applies to the universe as a whole, not to a chemical reaction in isolation. A local decrease in entropy is always accompanied by an even higher increase in the entropy of the surroundings. The production of heat in a chemical reaction increases entropy because heat is associated with the motion of molecules. The combination of the decrease in entropy associated with the building of a macromolecule and the increase in entropy associated with heat always results in a net increase in entropy.
The key point here is that the maintenance of the high degree of function and organization of a single cell or a multicellular organism requires a constant input of energy. This input, as we have seen, comes either from the sun or from the energy stored in chemical compounds. This energy allows molecules to be built and other work to be carried out, but also leads to greater entropy in the surroundings.
Quick Check 1 Cold air has less entropy than hot air. The second law of thermodynamics states that entropy always increases. Do air conditioners violate this law?
Quick Check 1 Answer
No, because the second law of thermodynamics applies to the universe as a whole. This means that we have to consider not just the air in the room but the heat released to the outdoors as well. An air conditioner produces more hot air than cold air, and therefore total entropy increases, as described by the second law of thermodynamics.