A chemical reaction occurs when molecules interact.
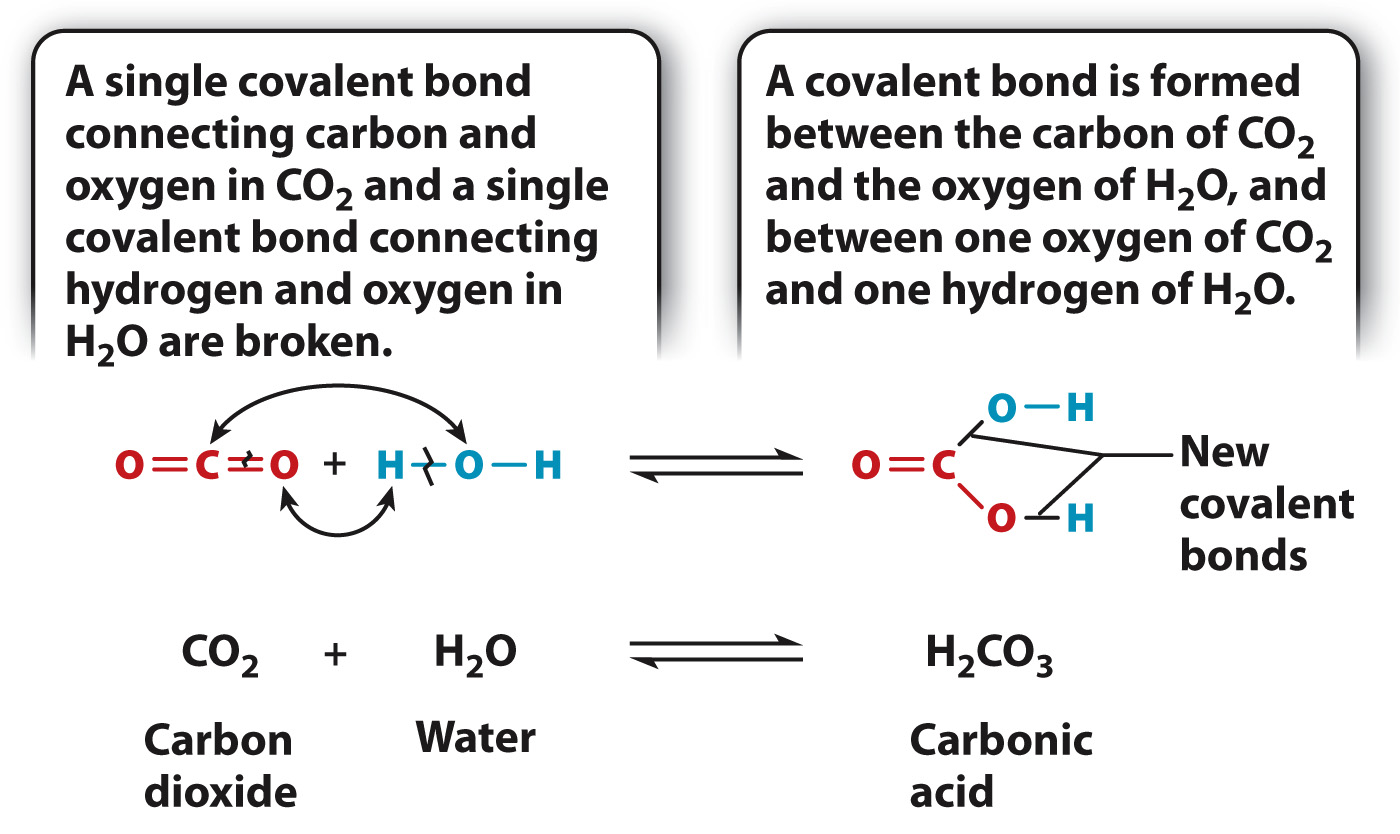
As we saw in Chapter 2, a chemical reaction is the process by which molecules, called reactants, are transformed into other molecules, called products. During a chemical reaction, atoms keep their identity, but the bonds linking the atoms change. For example, carbon dioxide (CO2) and water (H2O) can react to produce carbonic acid (H2CO3), as shown in Fig. 6.7.
121
This reaction is common in nature. For example, it occurs when carbon dioxide in the air enters ocean waters, and explains why the oceans are becoming more acidic with increasing carbon dioxide levels in the atmosphere. This reaction also takes place in the bloodstream. When cells break down glucose, they produce carbon dioxide. In animals, this carbon dioxide diffuses out of cells and into the blood. Rather than remaining dissolved in solution, most of the carbon dioxide is converted into carbonic acid by the preceding reaction. In an aqueous (watery) environment like the ocean or the blood, carbonic acid exists as bicarbonate ions (HCO3–) and protons (H+).
Most chemical reactions in cells are readily reversible: The products can react to form the reactants. For example, carbon dioxide and water react to form carbonic acid, and carbonic acid can dissociate to produce carbon dioxide and water. This reverse reaction also occurs in the blood, allowing carbon dioxide to be removed from the lungs or gills (Chapter 39). The reversibility of the reaction is indicated by a double arrow (Fig. 6.7).
The way the reaction is written defines forward and reverse reactions: A forward reaction proceeds from left to right and the reactants are located on the left side of the arrow; a reverse reaction proceeds from right to left and the reactants are located on the right side of the arrow.
The direction of a reaction can be influenced by the concentrations of reactants and products. For example, increasing the concentration of the reactants or decreasing the concentration of the products favors the forward reaction. This effect explains how many reactions in metabolic pathways proceed: The products of many reactions are quickly consumed by the next reaction, helping to drive the first reaction forward.