The hydrolysis of ATP is an exergonic reaction.
Let’s apply these concepts to a specific chemical reaction. Earlier, we introduced ATP, the molecule that drives many cellular processes using the chemical potential energy in its chemical bonds. ATP reacts with water to form ADP and inorganic phosphate, Pi (HPO42-
ATP + H2O → ADP + Pi
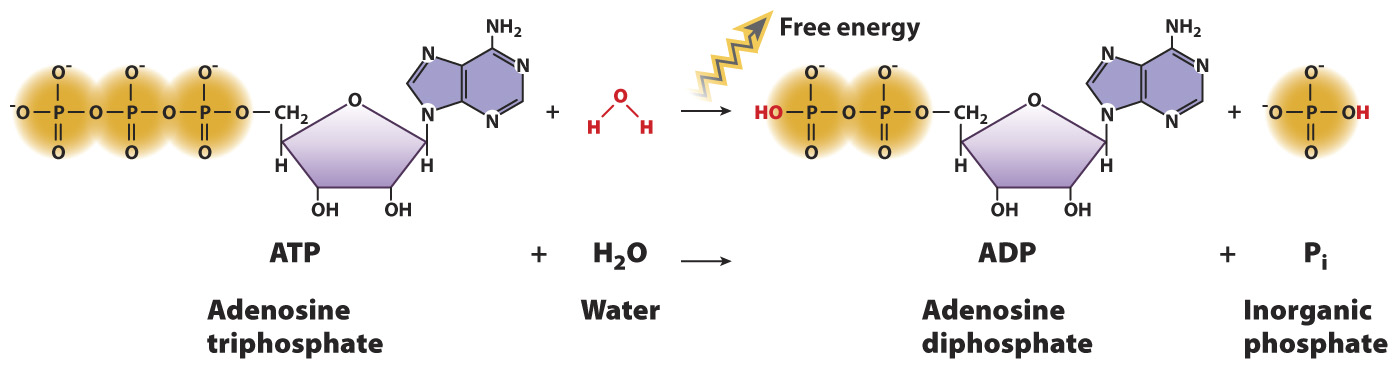
This is an example of a hydrolysis reaction, a chemical reaction in which a water molecule is split into a proton (H+) and a hydroxyl group (OH-). Hydrolysis reactions often break down polymers into their subunits, and in the process one product gains a proton and the other gains a hydroxyl group.
The reaction of ATP with water is an exergonic reaction because there is less free energy in the products compared to the reactants. The free energy difference can be explained by referring to the formula we derived in the last section. Recall that the phosphate groups of ATP are negatively charged at physiological pH and repel each other. ATP has three phosphate groups, and ADP has two. Therefore, ADP is more stable (contains less chemical energy in its bonds) than ATP, resulting in a negative value of ΔH. In addition, a single molecule of ATP is broken down into two molecules, ADP and Pi. Therefore, the reaction is also associated with an increase in entropy, or a positive value of ΔS. Since ΔG = ΔH – TΔS, ΔG is negative and the reaction is a spontaneous one that releases energy available to do work.
The free energy difference for ATP hydrolysis is approximately –7.3 kcal per mole (kcal/mol) of ATP. This value is influenced by several factors, including the concentration of reactants and products, the pH of the solution in which the reaction occurs, and the temperature and pressure. The value –7.3 kcal/mol is the value under standard laboratory conditions in which the concentrations of reactants and products are equal and pressure is held constant. In a cell, it is likely higher, on the order of –12 kcal/mol.
123
Keep in mind that the release of free energy during ATP hydrolysis comes from breaking weaker bonds (with more chemical energy) in the reactants and forming more stable bonds (with less chemical energy) in the products. The release of free energy then drives chemical reactions and other processes that require a net input of energy, as we discuss next.