Non-
If the conversion of reactant A into product B is spontaneous, the reverse reaction converting reactant B into product A is not. The ΔG’s for the forward and reverse reactions have the same absolute value but opposite signs. You might expect that the direction of the reaction would always be from A to B. However, in living organisms, not all chemical reactions are spontaneous. Anabolic reactions are a good example; they require an input of energy to drive them in the right direction. This raises the question: What drives non-
Energetic coupling is a process in which a spontaneous reaction (negative ΔG) drives a non-
For example, ATP hydrolysis can be used to drive a non-
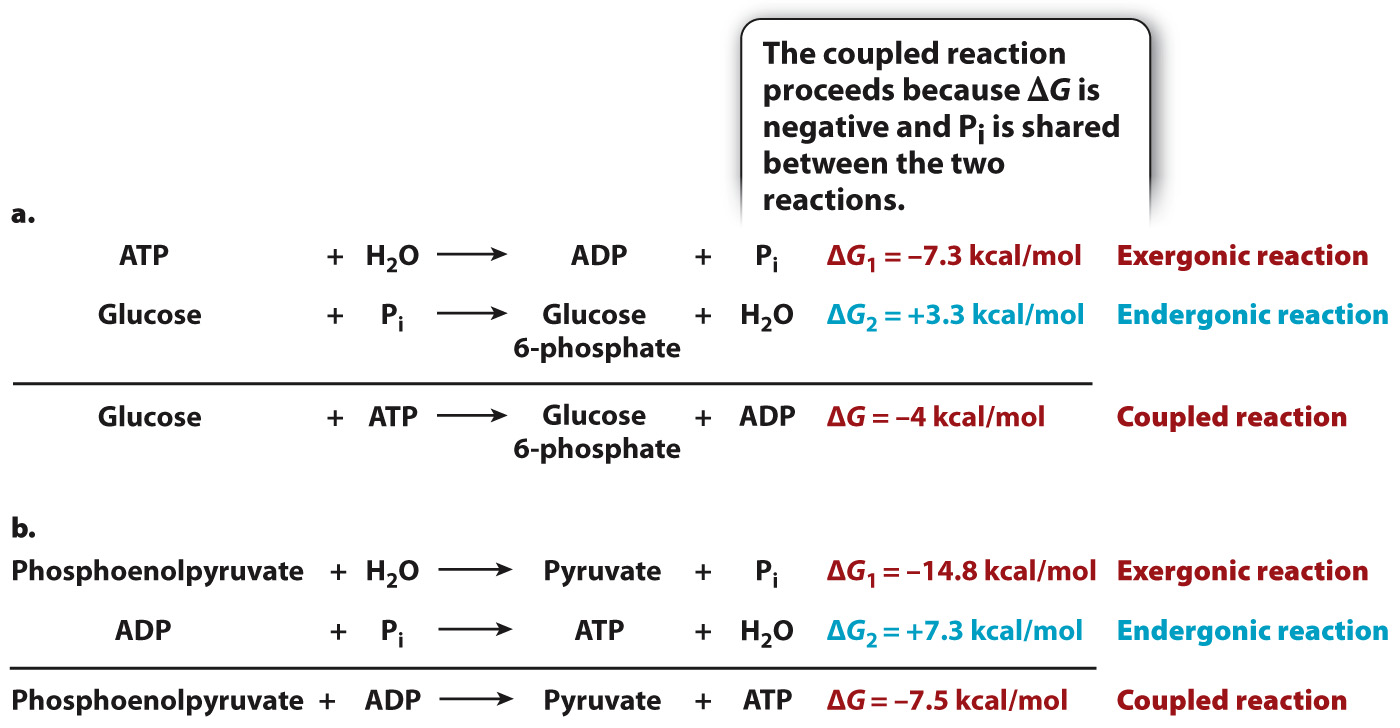
Following ATP hydrolysis, the cell needs to replenish its ATP so that it can carry out additional chemical reactions. The synthesis of ATP from ADP and Pi is an endergonic reaction with a positive ΔG, requiring an input of energy. In some cases, exergonic reactions can drive the synthesis of ATP by energetic coupling (Fig. 6.11b). The sum of the ΔG’s of the two reactions is negative and the reactions share a phosphate group, allowing the two reactions to proceed.
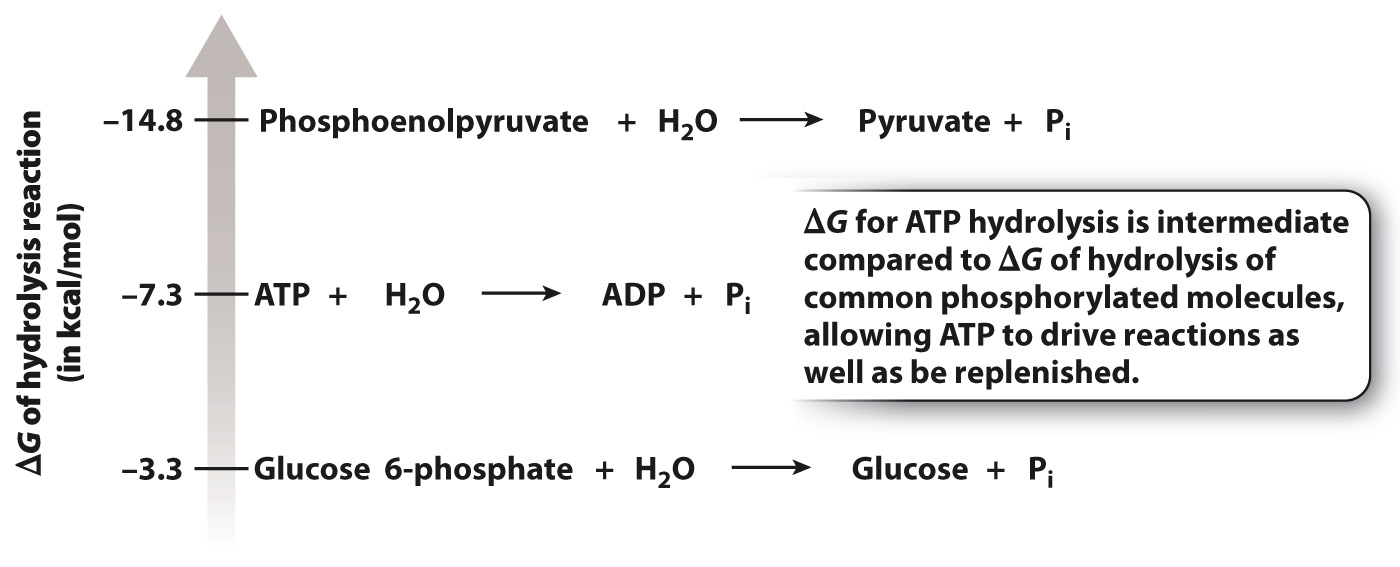
Like ATP, other phosphorylated molecules can be hydrolyzed, releasing free energy. Hydrolysis reactions can be ranked by their free energy differences (ΔG). Fig. 6.12 shows that ATP hydrolysis has an intermediate free energy difference compared with the free energy difference for the hydrolysis of other common phosphorylated molecules. Those reactions that have a ΔG more negative than that of ATP hydrolysis transfer a phosphate group to ADP by energetic coupling, and those reactions that have a ΔG less negative than that of ATP hydrolysis receive a phosphate group from ATP by energetic coupling. Therefore, ADP is an energy acceptor and ATP an energy donor. The ATP–
124