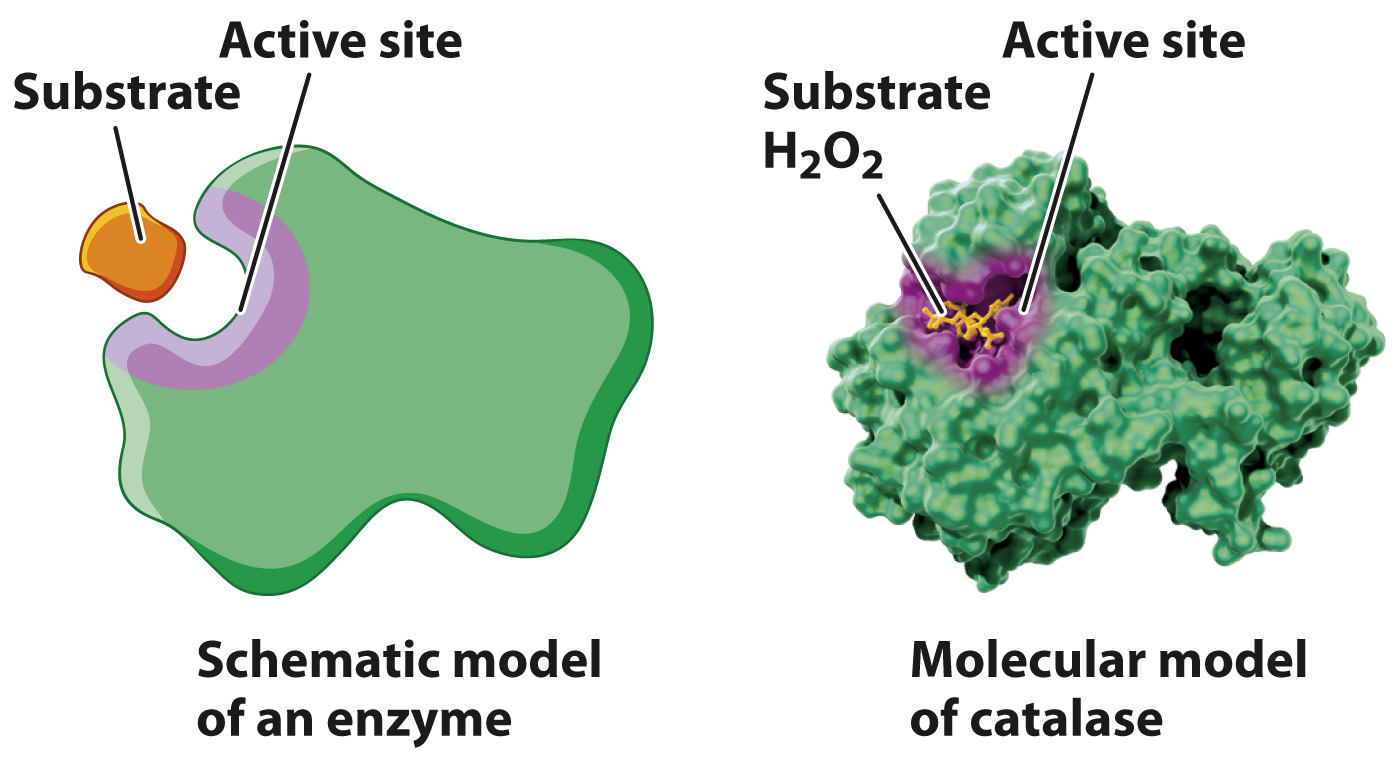
Enzymes form a complex with reactants and products.
An important characteristic of enzymes is that, as catalysts, they participate in a chemical reaction but are not consumed in the process. In other words, they emerge unchanged from a chemical reaction, ready to catalyze the same reaction again. How do enzymes increase the reaction rate without being consumed? The answer is that enzymes form a complex with the reactants and products.
In a chemical reaction catalyzed by an enzyme, the reactant is often referred to as the substrate. In such a reaction, the substrate (S) is converted to a product (P):
S ⇋ P
In the presence of an enzyme (E), the substrate first forms a complex with the enzyme (enzyme–
S + E ⇋ ES ⇋ EP ⇋ E + P
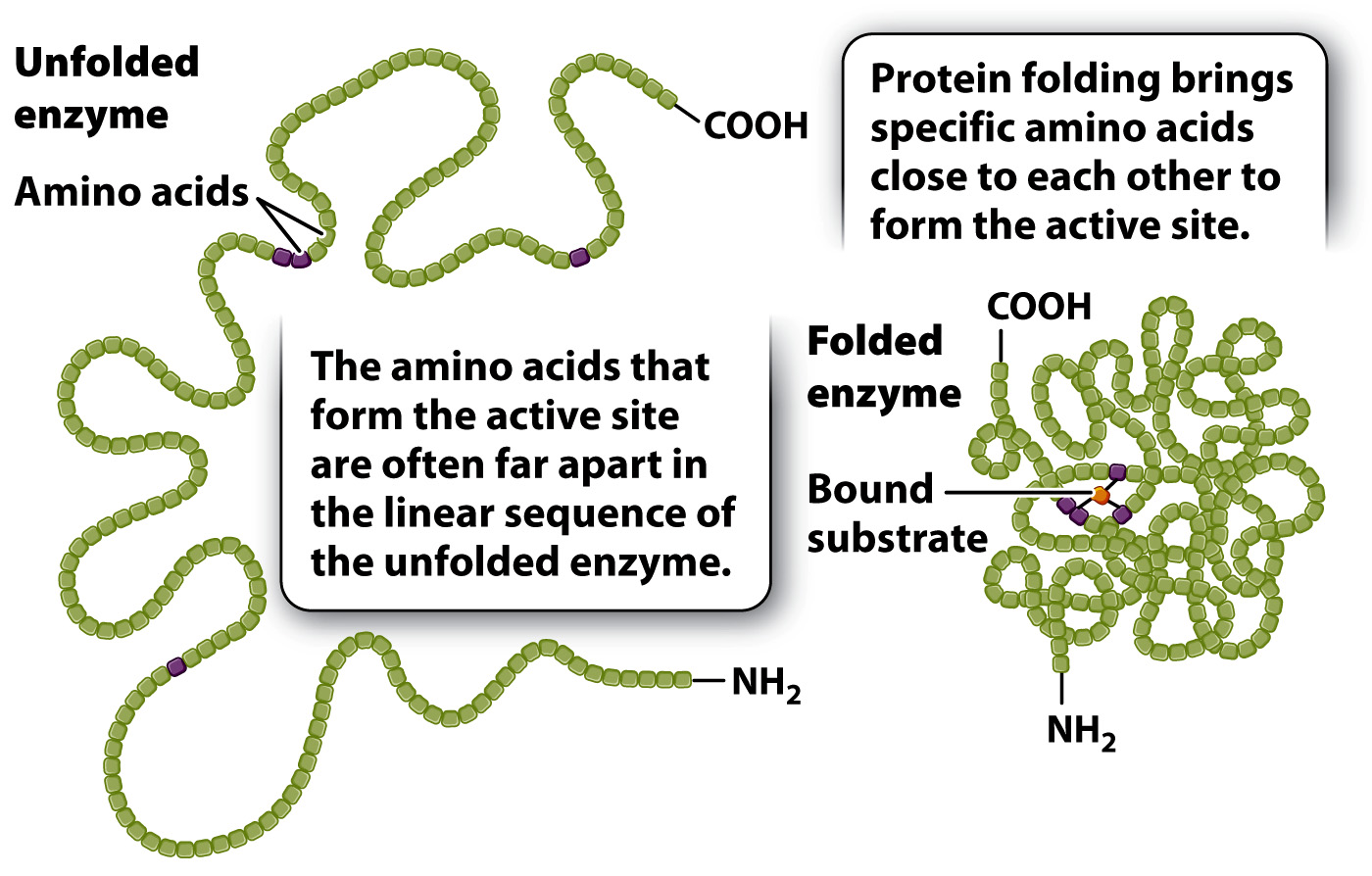
The formation of this complex is critical for accelerating the rate of a chemical reaction. Recall from Chapter 4 that proteins adopt three-
Enzymes also reduce the energy of activation by positioning two substrates to react. The formation of the enzyme–
The size of the active site is extremely small compared with the size of the enzyme. If only a small fraction of the enzyme is necessary for the catalysis of a reaction, why are enzymes so large? Of the many amino acids that form the active site, only a few actively contribute to catalysis. Each of these amino acids has to occupy a very specific spatial position to align with the correct reactive group on the substrate. If the few essential amino acids were part of a short peptide, the alignment of chemical groups between the peptide and the substrate would be difficult or even impossible because the length of the bonds and the bond angles in the peptide would constrain its three-
126
The formation of a complex between enzymes and substrates can be demonstrated experimentally, as illustrated in Fig. 6.16.
HOW DO WE KNOW?
FIG. 6.16
Do enzymes form complexes with substrates?
BACKGROUND The idea that enzymes form complexes with substrates to catalyze a chemical reaction was first proposed in 1888 by the Swedish chemist Svante Arrhenius. One of the earliest experiments that supported this idea was performed by American chemist Kurt Stern in the 1930s. He studied an enzyme called catalase, which is very abundant in animal and plant tissues. In the conclusion of his paper, he wrote, “It remains to be seen to which extent the findings of this study apply to enzyme action in general.” Therefore, another experiment that analyzes a different enzyme and technique are described here.
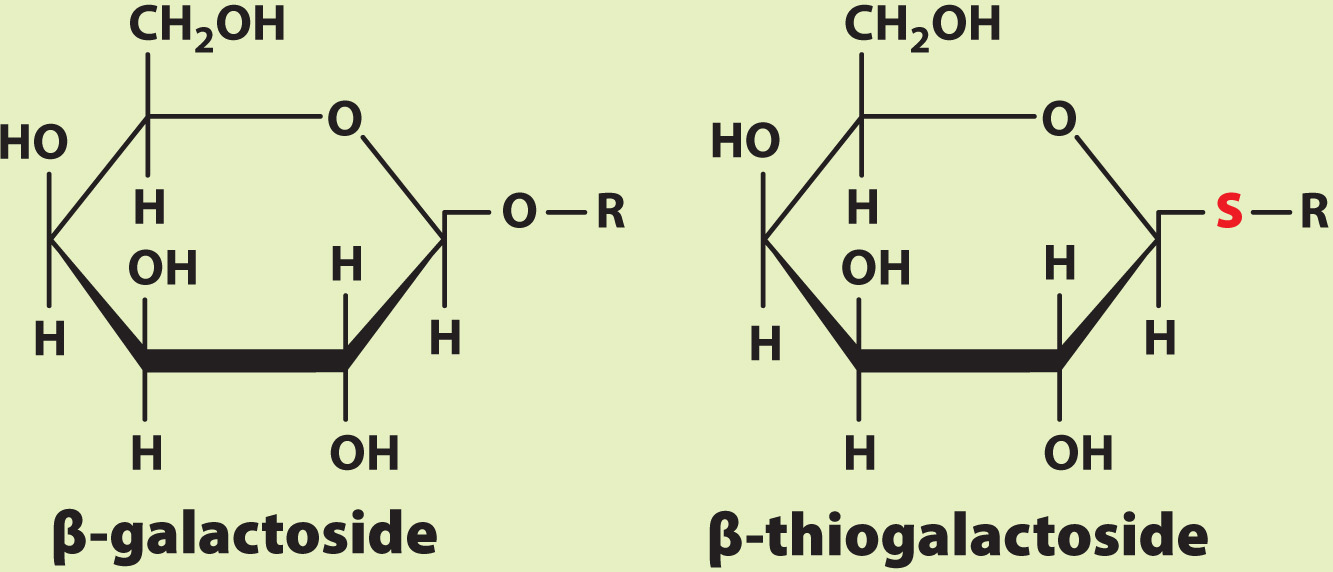
The enzyme β-galactosidase catalyzes the cleavage of the glycosidic bond that links galactose to glucose in the disaccharide lactose. Lactose belongs to a family of molecules called β-galactosides since it contains a galactose unit attached to the rest of the molecule by a glycosidic bond. In a related compound called β-thiogalactoside, the oxygen atom in the glycosidic bond is replaced by sulfur. The enzyme β-galactosidase binds β-thiogalactoside but cannot cleave or release it.
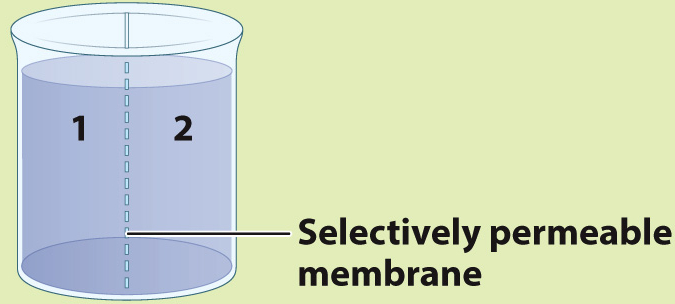
METHOD A container is separated into two compartments by a selectively permeable membrane. The membrane is permeable to β-galactoside and β-thiogalactoside, but not permeable to the enzyme.
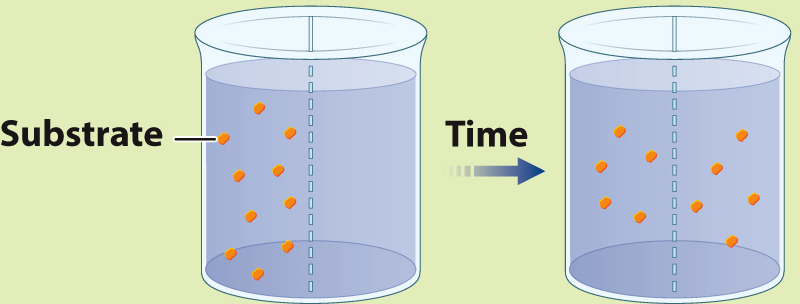
EXPERIMENT 1 Radioactively labeled β-thiogalactoside (S) is added to compartment 1 and the movement of S is followed by measuring the level of radioactivity in the two compartments.
RESULT Over time, the level of radioactivity is the same in the two compartments.
EXPERIMENT 2 Radioactively labeled β-thiogalactoside (S) is added to compartment 1, enzyme (E) is added to compartment 2, and the movement of S is followed by measuring the level of radioactivity in the two compartments.
RESULT Over time, the level of radioactivity is greater in compartment 2 than in compartment 1.
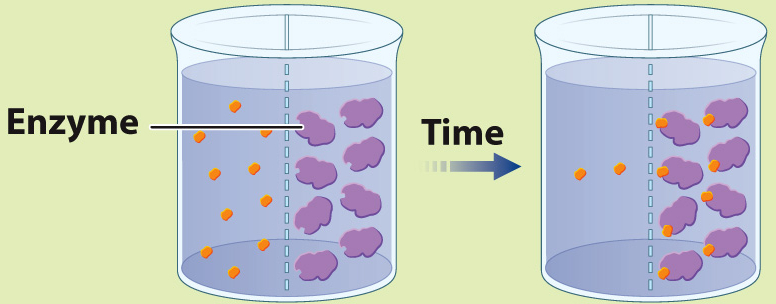
CONCLUSION These results can be explained if the substrate diffuses from compartment 1 to compartment 2, forms a complex with the enzyme, and is not released because the enzyme cannot catalyze the conversion of substrate to product. In other words, E and S form a complex.
SOURCES Adapted from Doherty, D. G., and F. Vaslow. 1952. “Thermodynamic Study of an Enzyme–