ATP is a readily accessible form of cellular energy.
The chemical energy carried in carbohydrates, lipids, and proteins is harnessed by cells to do work. But cells do not use this energy all at once. Instead, through the series of chemical reactions described in Chapter 7, they package this energy into a chemical form that is readily accessible to the cell. One form of chemical energy is adenosine triphosphate, or ATP, shown in Fig. 6.4. The chemical energy in the bonds of ATP is used in turn to drive many cellular processes, such as muscle contraction, cell movement, and membrane pumps. In this way, ATP serves as a go-
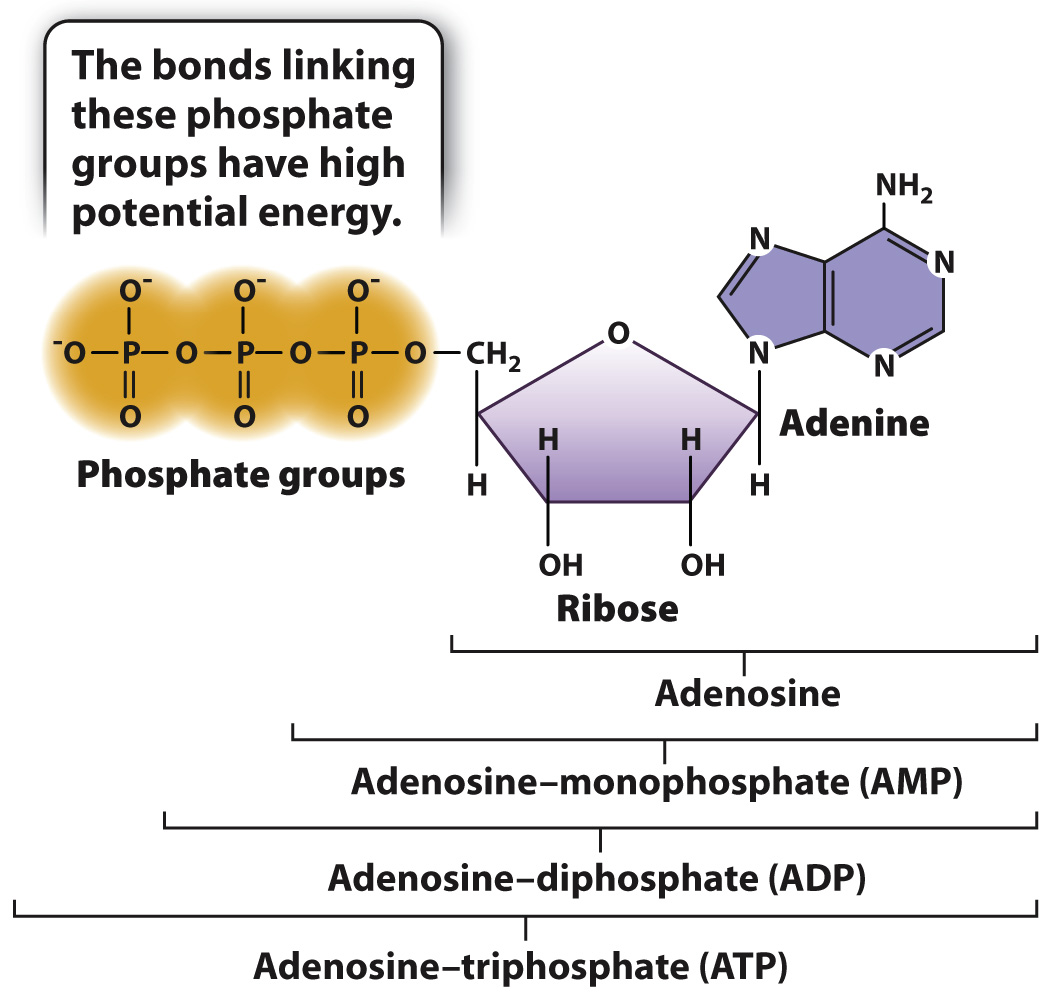
ATP is composed in part of adenosine, which is made up of the base adenine and the five-
The chemical energy of ATP is held in the bonds connecting the phosphate groups. At physiological pH, these phosphate groups are negatively charged and have a tendency to repel each other. The chemical bonds connecting the phosphate groups therefore store chemical energy. This energy is released when new, more stable bonds are formed that contain less chemical energy (section 6.4). The released energy in turn can be harnessed to power the work of the cell.