The electron transport chain transfers electrons and pumps protons.
Electrons donated by NADH and FADH2 are transported along a series of four large protein complexes that form the electron transport chain (complexes I to IV). These are shown in Fig. 7.10. These membrane proteins are embedded in the mitochondrial inner membrane (see Fig. 7.6). The inner mitochondrial membrane contains one of the highest concentrations of proteins found in eukaryotic membranes.
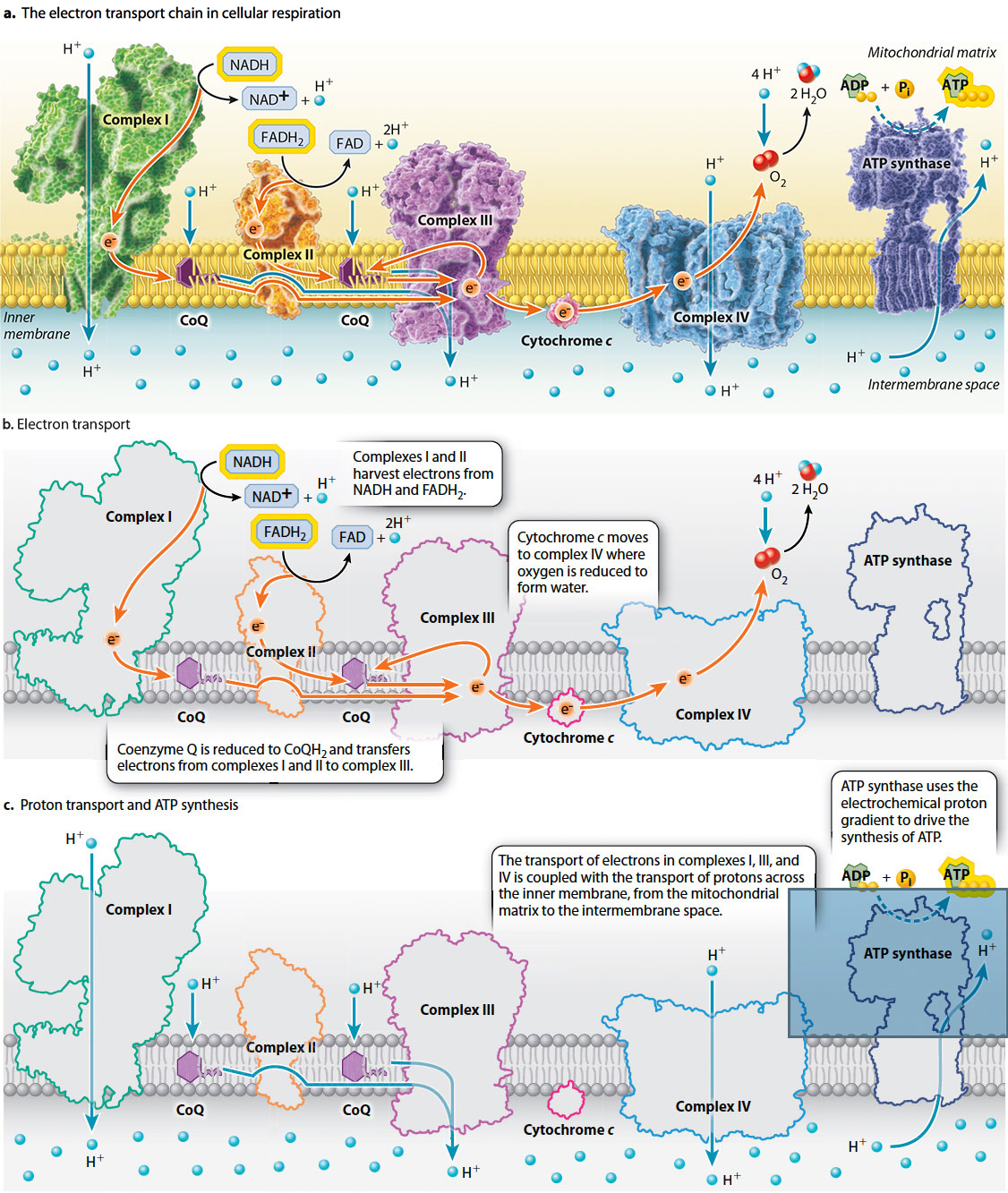
Electrons enter the electron transport chain at either complex I or II. Electrons donated by NADH enter through complex I, and electrons donated by FADH2 enter through complex II. (Complex II is the same enzyme that catalyzes step 6 in the citric acid cycle.) These electrons are transported through either complex I or II to complex III and then through complex IV.
Within each protein complex of the electron transport chain, electrons are passed from electron donors to electron acceptors. Each donor and acceptor is a redox couple, consisting of an oxidized and a reduced form of a molecule. The electron transport chain contains many of these redox couples. When oxygen accepts electrons at the end of the electron transport chain, it is reduced to form water:
O2 + 4e– + 4H+ → 2H2O
This reaction is catalyzed by complex IV.
141
142
Electrons also must be transported between the four complexes (Fig. 7.10). Coenzyme Q (CoQ), also called ubiquinone, accepts electrons from both complexes I and II. In this reaction, two electrons and two protons are transferred to CoQ from the mitochondrial matrix, forming CoQH2. Once CoQH2 is formed, it diffuses in the inner membrane to complex III. In complex III, electrons are transferred from CoQH2 to cytochrome c and protons are released into the intermembrane space. When it accepts an electron, cytochrome c is reduced, diffuses in the intermembrane space, and passes the electron to complex IV.
These electron transfer steps are each associated with the release of energy as electrons are passed from the reduced electron carriers NADH and FADH2 to the final electron acceptor, oxygen. Some of this energy is used to reduce the next carrier in the chain, but in complexes I, III, and IV, some of it is used to pump protons (H+) across the inner mitochondrial membrane, from the mitochondrial matrix to the intermembrane space (Fig. 7.10). Thus, the transfer of electrons through complexes I, III, and IV is coupled with the pumping of protons. The result is an accumulation of protons in the intermembrane space.
Quick Check 4 Animals breathe in air that contains more oxygen than the air they breathe out. Where is oxygen consumed?
Quick Check 4 Answer
Oxygen is consumed in cellular respiration. Oxygen is the final electron acceptor in the electron transport chain and is converted to water.