Fermentation extracts energy from glucose in the absence of oxygen.
Pyruvate has many possible fates in the cell. In the absence of oxygen, it can be broken down by fermentation, which does not rely on oxygen or any other electron acceptor. Fermentation is accomplished through a wide variety of metabolic pathways that extract energy from fuel molecules such as glucose. Fermentation pathways are important for anaerobic organisms that live without oxygen, as well as some organisms such as yeast that favor fermentation over oxidative phosphorylation, even in the presence of oxygen. It is also sometimes used in aerobic organisms when oxygen cannot be delivered fast enough to meet the cell’s metabolic needs, as in exercising muscle.
Recall that during glycolysis, glucose is oxidized to form pyruvate, and NAD+ is reduced to form NADH. For glycolysis to continue, NADH must be oxidized to NAD+. If that did not happen, glycolysis would grind to a halt. In the presence of oxygen, NAD+ is regenerated when NADH donates its electrons to the electron transport chain. In the absence of oxygen during fermentation, NADH is oxidized to NAD+ when pyruvate or a derivative of pyruvate is reduced.
There are many fermentation pathways, especially in bacteria. Two of the major pathways are lactic acid fermentation and ethanol fermentation (Fig. 7.14). Lactic acid fermentation occurs in animals and bacteria. During lactic acid fermentation, electrons from NADH are transferred to pyruvate to produce lactic acid and NAD+ (Fig. 7.14a). The overall chemical reaction is written as follows:
Glucose + 2 ADP + 2 Pi → 2 lactic acid + 2 ATP + 2H2O
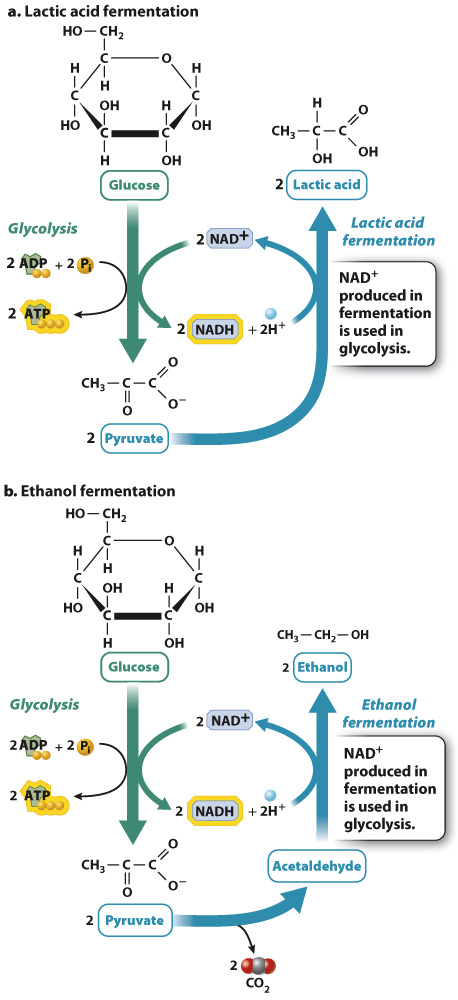
Ethanol fermentation occurs in plants and fungi. During ethanol fermentation, pyruvate releases carbon dioxide to form acetaldehyde, and electrons from NADH are transferred to acetaldehyde to produce ethanol and NAD+ (Fig. 7.14b). The overall chemical reaction is written as follows:
Glucose + 2 ADP + 2 Pi → 2 ethanol + 2CO2 + 2 ATP + 2H2O
146
In both fermentation pathways, NADH is oxidized to NAD+. However, NADH and NAD+ do not appear in the overall chemical equations because there is no net production or loss of either molecule. NAD+ molecules that are reduced during glycolysis are oxidized when lactic acid or ethanol is formed.
The breakdown of a molecule of glucose by fermentation yields only two molecules of ATP. The energetic gain is relatively small compared with the total yield of aerobic respiration because the end products, lactic acid and ethanol, are not fully oxidized and still contain a large amount of chemical energy in their bonds. The modest yield explains why organisms that produce ATP by fermentation must consume a large quantity of fuel molecules to power the cell.
Quick Check 6 Bread making involves ethanol fermentation and typically uses yeast, sugar, flour, and water. Why are yeast and sugar used?
Quick Check 6 Answer
Yeast cells are eukaryotes. In bread making, yeast can use sugar as a food source for ethanol fermentation. The carbon dioxide produced in the process causes the bread to rise. The ethanol is removed in the baking process.