Fatty acids and proteins are useful sources of energy.
In addition to carbohydrates, lipids are also a good source of energy. We know this from common experience. Butter, oils, ice cream, and the like all contain lipids and are high in calories, which are units of energy. We can also infer that lipids are a good source of energy from their chemical structure. Recall from Chapter 2 that a type of fat called triacylglycerol is composed of three fatty acid molecules bound to a glycerol backbone. These fatty acid molecules are rich in carbon–
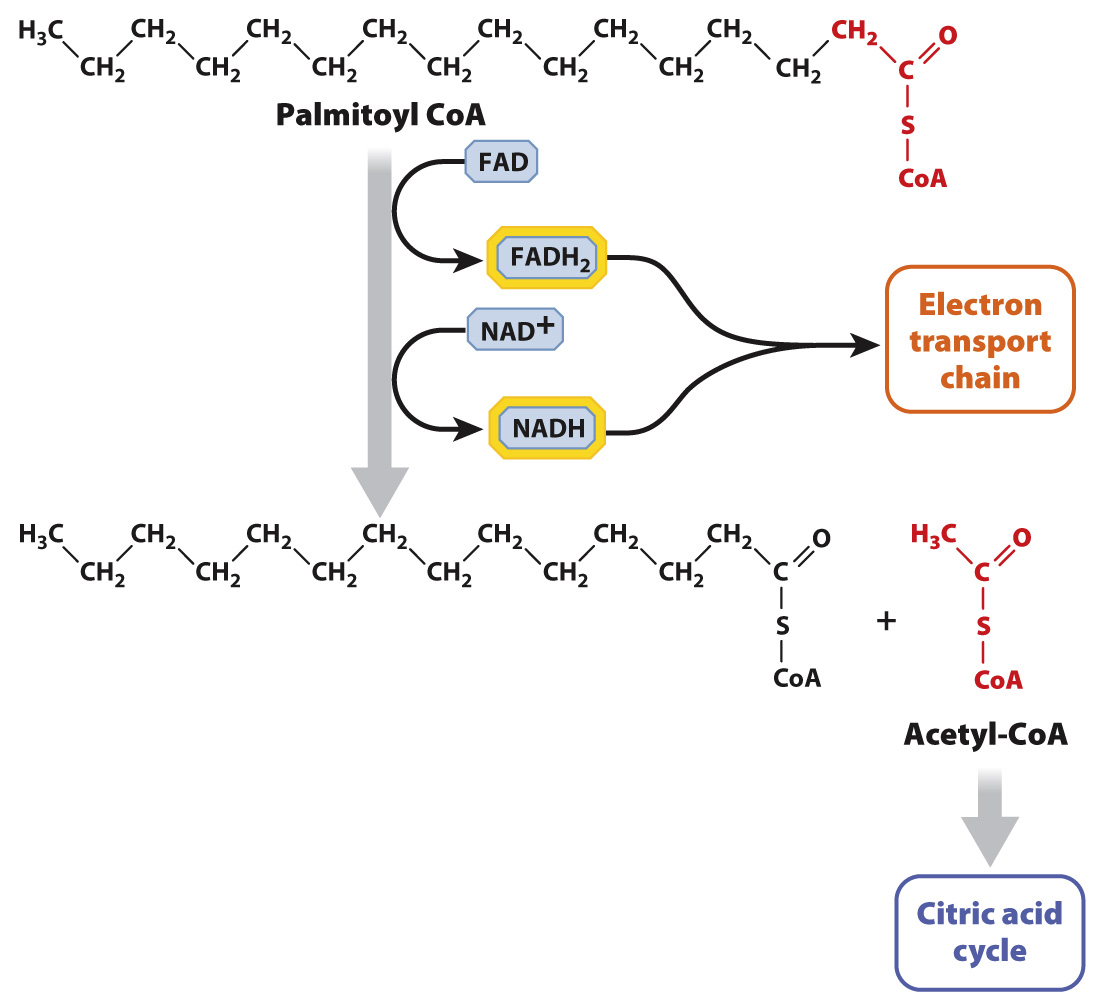
Following a meal, the small intestine very quickly absorbs triacylglycerols, which are then transported by the bloodstream and either consumed or stored in fat (adipose) tissue. Triacylglycerols are broken down inside cells to glycerol and fatty acids. Then, the fatty acids themselves are shortened by a series of reactions that sequentially remove two carbon units from their ends (Fig. 7.18). This process is called β-(beta-
149
The oxidation of fatty acids produces a large amount of ATP. For example, the complete oxidation of a molecule of palmitic acid, a fatty acid containing 16 carbons, yields about 106 molecules of ATP. By contrast, glycolysis yields just 2 molecules of ATP, and the complete oxidation of a glucose molecule produces about 32 molecules of ATP (Table 7.1). Fatty acids therefore are a useful and efficient source of energy, but they cannot be used by all tissues of the body. Notably, the brain and red blood cells depend primarily on glucose for energy.
Proteins, like fatty acids, are a source of chemical energy that can be broken down, if necessary, to power the cell. Proteins are typically first broken down to amino acids, some of which can then enter glycolysis and others the citric acid cycle.