The intracellular level of ATP is a key regulator of cellular respiration.
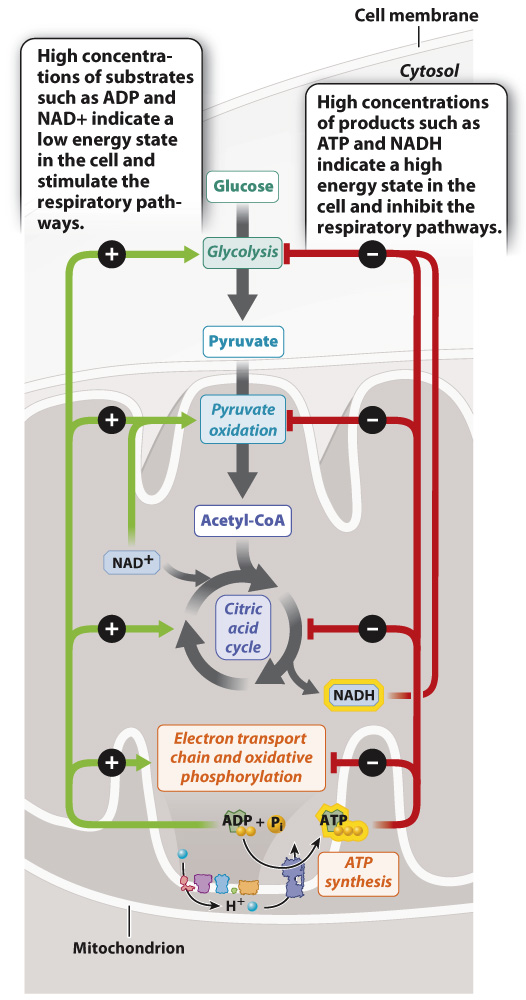
FIG. 7.19 Regulation of cellular respiration. Cellular respiration is inhibited by its products, including ATP and NADH, and activated by its substrates, including ADP and NAD+.
ATP is the key end product of cellular respiration, holding in its bonds energy that can be used for all kinds of cellular processes. ATP is constantly being turned over in a cell, broken down to ADP and Pi to supply the cell’s energy needs, and re-synthesized by fermentation and cellular respiration. The level of ATP inside a cell can therefore be an indicator of how much energy a cell has available. When ATP levels are high, the cell has a high amount of free energy and is poised to carry out cellular processes. In this case, pathways that generate ATP are slowed, or down-regulated. By contrast, when ATP levels are low, the cell activates, or up-regulates, pathways that lead to ATP synthesis. Other intermediates of cellular respiration, such as NADH, have a similar effect in that high NAD+ levels stimulate cellular respiration, whereas high NADH levels inhibit it (Fig. 7.19).
How is this kind of coordinated response of the cell possible? The cell uses several mechanisms, one of which is the regulation of enzymes that control key steps of the pathway. One of these key reactions is reaction 3 of glycolysis. In this reaction, fructose 6-phosphate is converted to fructose 1,6-bisphosphate, and a molecule of ATP is consumed. This is a key step in glycolysis because it is highly endergonic and irreversible. As a result, it is considered a “committed” step and is subject to tight control. This reaction is catalyzed by the enzyme phosphofructokinase-1 (PFK-1), which can be thought of as a metabolic valve that regulates the rate of glycolysis.
PFK-1 is an allosteric enzyme with many activators and inhibitors (Fig. 7.20). Recall from Chapter 6 that an allosteric enzyme changes its shape and activity in response to the binding of molecules at a site other than the active site. ADP and AMP are allosteric activators of PFK-1. When ADP and AMP are abundant, one or the other binds to the enzyme and causes the enzyme’s shape to change. The shape change activates the enzyme, increasing the rate of glycolysis and the synthesis of ATP. When ATP is in abundance, it binds to the same site on the enzyme as ADP and AMP, but in this case binding inhibits the enzyme’s catalytic activity. As a result, glycolysis and the rate of ATP production slow down.
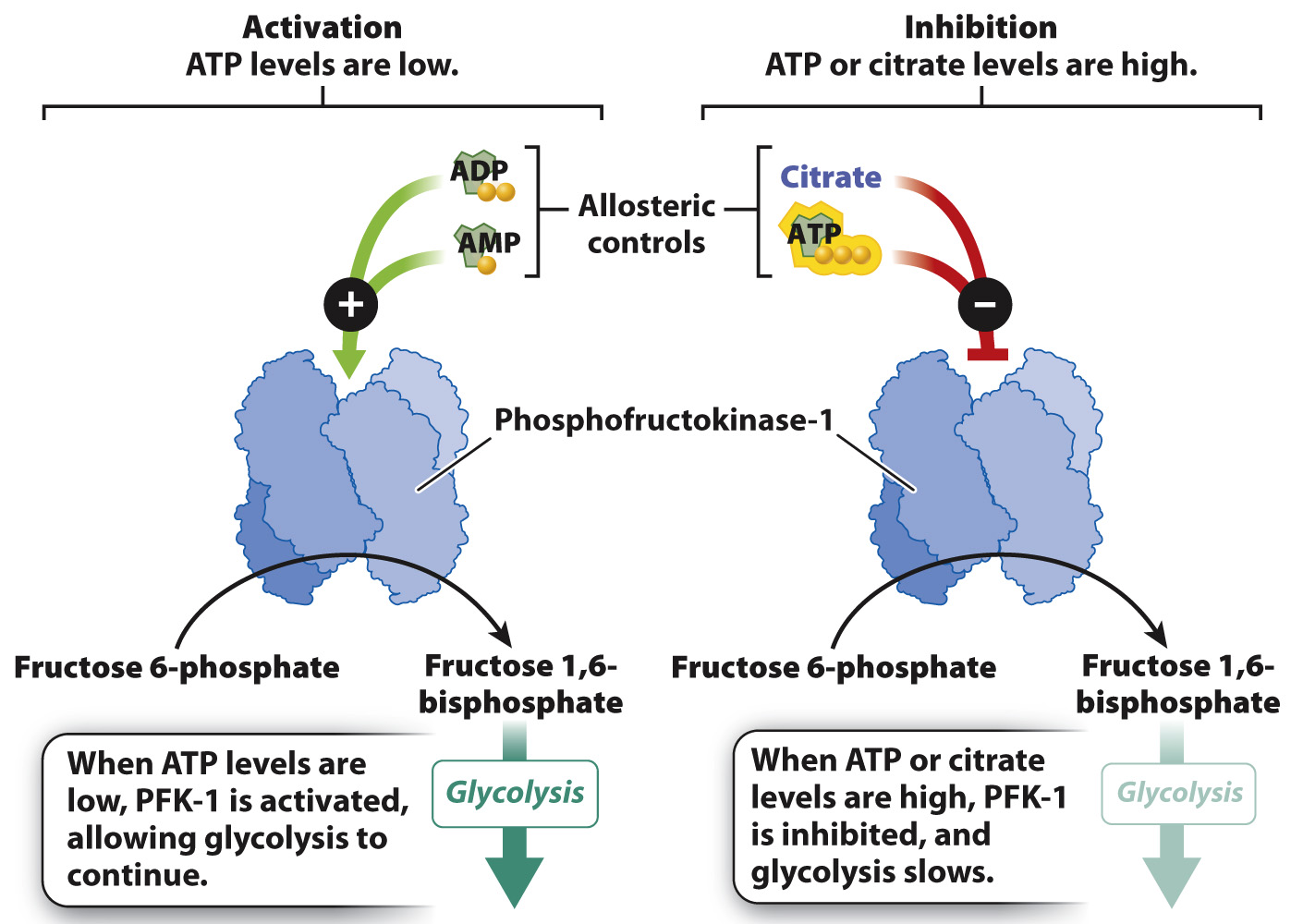
FIG. 7.20 Regulation of PFK-1.The regulation of the glycolytic enzyme phosphofructokinase-1 (PFK-1) is an example of integrated metabolic control.
PFK-1 is also regulated by one of its downstream products, citrate, an intermediate in the citric acid cycle (see Fig. 7.8). Citrate acts as an allosteric inhibitor of the enzyme, slowing its activity. High levels of citrate indicate that is not being consumed by the citric acid cycle and glucose breakdown can be slowed. The role of citrate in controlling glycolysis illustrates the coordinated regulation of glycolysis and the citric acid cycle.

