Cellular respiration uses chemical energy stored in molecules such as carbohydrates and lipids to produce ATP.
Cellular respiration is a series of catabolic reactions that converts the energy stored in food molecules, such as glucose, into the energy stored in ATP, and produces carbon dioxide as a waste or by-
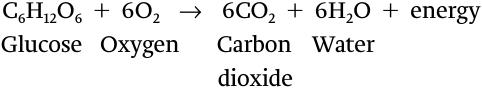
In Chapter 6, we saw that molecules such as carbohydrates and lipids have a large amount of potential energy in their chemical bonds. In contrast, molecules like carbon dioxide and water have less potential energy in their bonds. Cellular respiration releases a large amount of energy because the sum of the potential energy in all of the chemical bonds of the reactants (glucose and oxygen) is higher than that of the products (carbon dioxide and water). The maximum amount of free energy—
The overall reaction for cellular respiration helps us focus on the starting reactants, final products, and release of energy. However, it misses the many intermediate steps that take place as the cell catabolizes glucose. Tossing a match into the gas tank of a car releases a tremendous amount of energy in the form of an explosion, but this energy is not used to do work. Instead, it is released as light and heat. Similarly, if all the energy stored in glucose were released at once, most of it would be released as heat and the cell would not be able to harness it to do work.
In cellular respiration, energy is released gradually in a series of chemical reactions (Fig. 7.1). This allows some of this energy to be used to form ATP. On average, 32 molecules of ATP are produced from the aerobic respiration of a single molecule of glucose. The energy needed (ΔG of the reaction) to form one mole of ATP from ADP and Pi is at least 7.3 kcal. Thus, cellular respiration harnesses at least 32 × 7.3 = 233.6 kcal of energy in ATP for every mole of glucose that is broken down in the presence of oxygen.
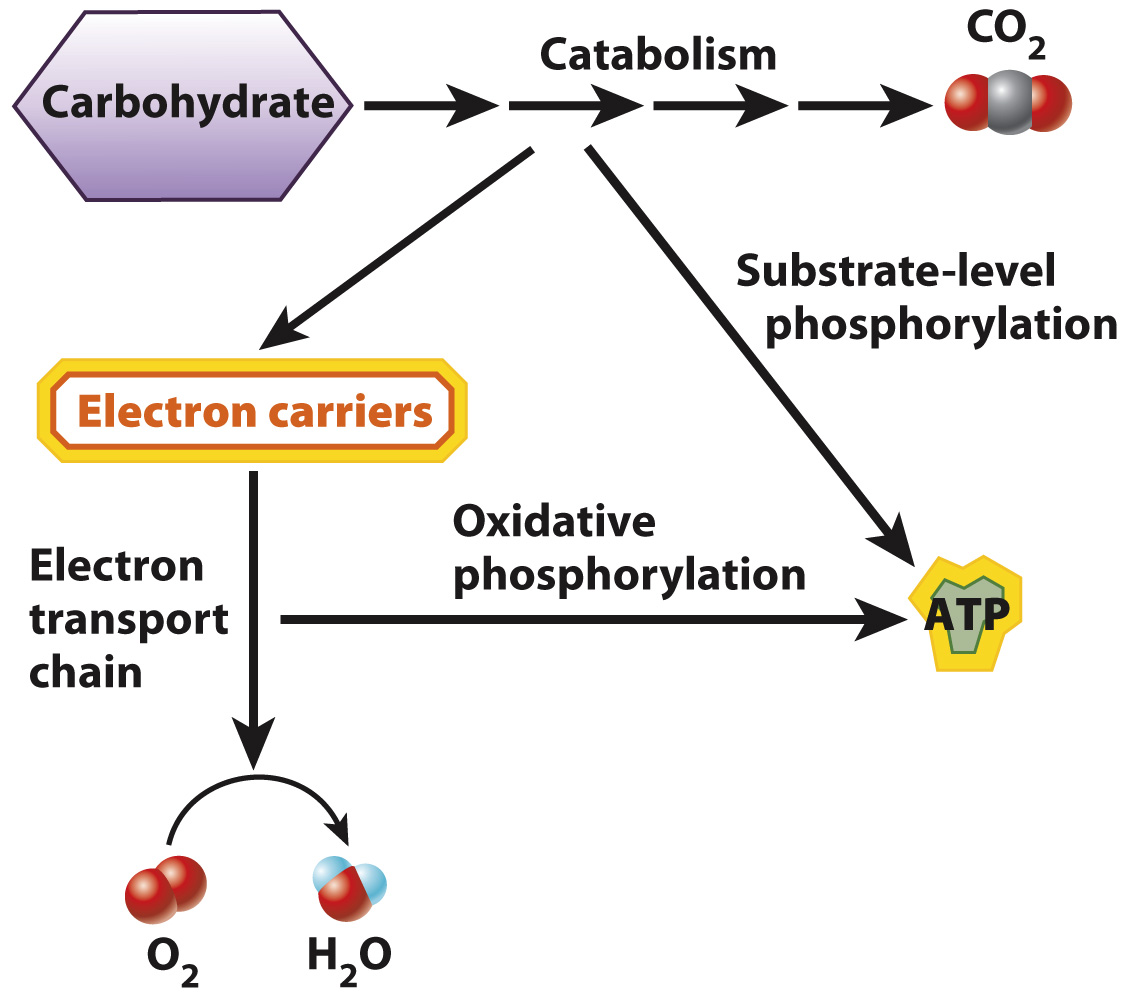
About 34% of the total energy released by aerobic respiration is harnessed in the form of ATP (233.6/686 = 34%), with the remainder of the energy given off as heat. This degree of efficiency compares favorably with that of a gasoline engine, which operates with an efficiency of about 25%.
133