Redox reactions play a central role in cellular respiration.
Chemical reactions in which electrons are transferred from one atom or molecule to another are referred to as oxidation–
We can illustrate oxidation–
NAD+ + 2e– + H+ → NADH
FAD + 2e– + 2H+ → FADH2
Here, NAD+ and FAD accept electrons, resulting in the production of their reduced forms, NADH and FADH2. Note that in redox reactions involving organic molecules such as NAD+ or FAD, the gain (or loss) of electrons is often accompanied by the gain (or loss) of protons (H+). As a result, reduced molecules can easily be recognized by an increase in C–
In their reduced forms, NADH and FADH2 can donate electrons. The oxidation of NADH and FADH2 allows electrons (and energy) to be transferred to the electron transport chain:
NADH → NAD+ + 2e– + H+
FADH2 → FAD + 2e– + 2H+
In addition, these reactions produce NAD+ and FAD, which can then accept electrons from the breakdown of fuel molecules. In this way, electron carriers act as shuttles, transferring electrons derived from the oxidation of fuel molecules such as glucose to the electron transport chain.
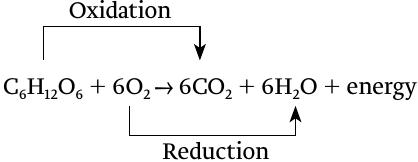
Cellular respiration can itself be understood as a redox reaction, even though it consists of many steps. In aerobic respiration, glucose is oxidized, releasing carbon dioxide, and at the same time oxygen is reduced, forming water:
134
Let’s first consider the oxidation reaction (Fig. 7.2a). In glucose, there are many C–
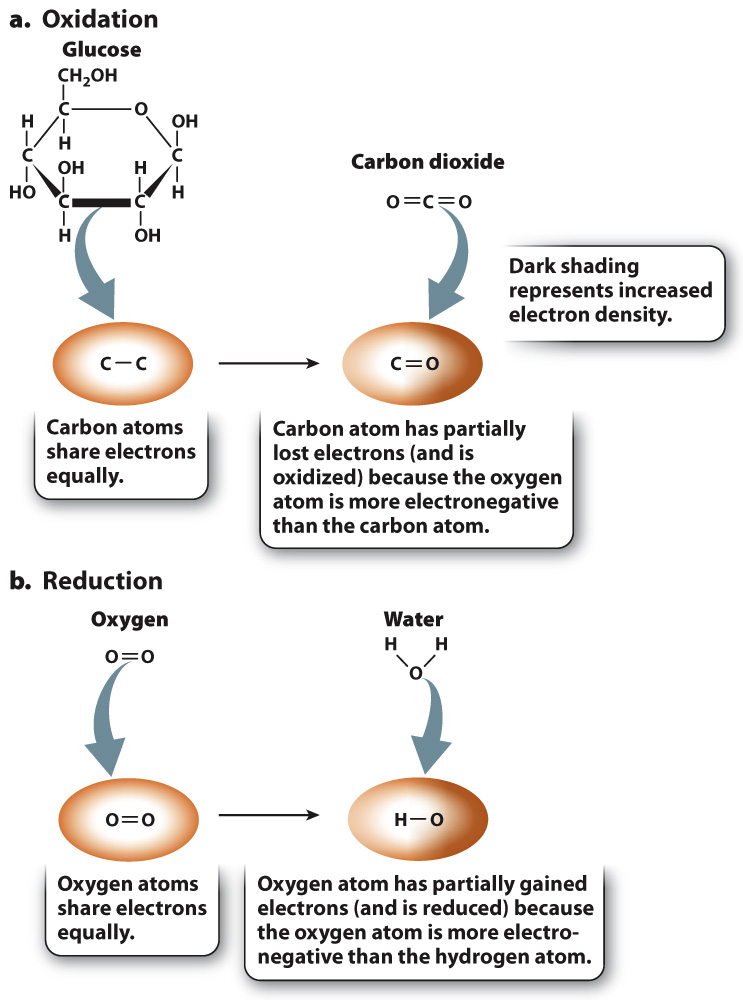
Let’s now consider the reduction reaction (Fig. 7.2b). In oxygen gas, electrons are shared equally between two oxygen atoms. In water, the electrons that are shared between hydrogen and oxygen are more likely to be found near oxygen because oxygen is more electronegative than hydrogen. As a result, oxygen has partially gained electrons and is reduced.
135
Glucose is a good electron donor because its oxidation to carbon dioxide releases a lot of energy, whereas oxygen is a good electron acceptor because it has a high affinity for electrons. In cellular respiration, glucose is not oxidized all at once to carbon dioxide. Instead, it is oxidized slowly in a series of reactions, some of which are redox reactions. In this way, energy is released in a controlled manner. Some of this energy can be used to synthesize ATP directly and some of it is stored temporarily in reduced electron carriers and then used to generate ATP by oxidative phosphorylation.
Quick Check 1 For each of the following pairs of molecules, indicate which member of the pair is reduced and which is oxidized, and which has more chemical energy and which has less chemical energy: NAD+/NADH; FAD/FADH2; CO2/C6H12O6.
Quick Check 1 Answer
The reduced molecules are NADH, FADH2, and C6H12O6, and the oxidized molecules are NAD+, FAD, and CO2. The reduced forms have more chemical energy than their corresponding oxidized forms.