The photosynthetic electron transport chain connects two photosystems.
In many ways, water is an ideal source of electrons for photo-
163
If you follow the flow of electrons from water through both photosystems and on to NADP+, as shown in Fig. 8.13, you can see a large increase in energy as the electrons pass through each of the two photosystems. You can also see that at every other step along the photosynthetic electron transport chain there is a small decrease in energy. This decrease in energy indicates that these are exergonic reactions (Chapter 6) and thus explains why electrons move in one “direction” through the series of redox reactions that make up the photosynthetic electron transport chain. To run these reactions in the opposite direction would require an input of energy. Because the overall energy trajectory has an up-
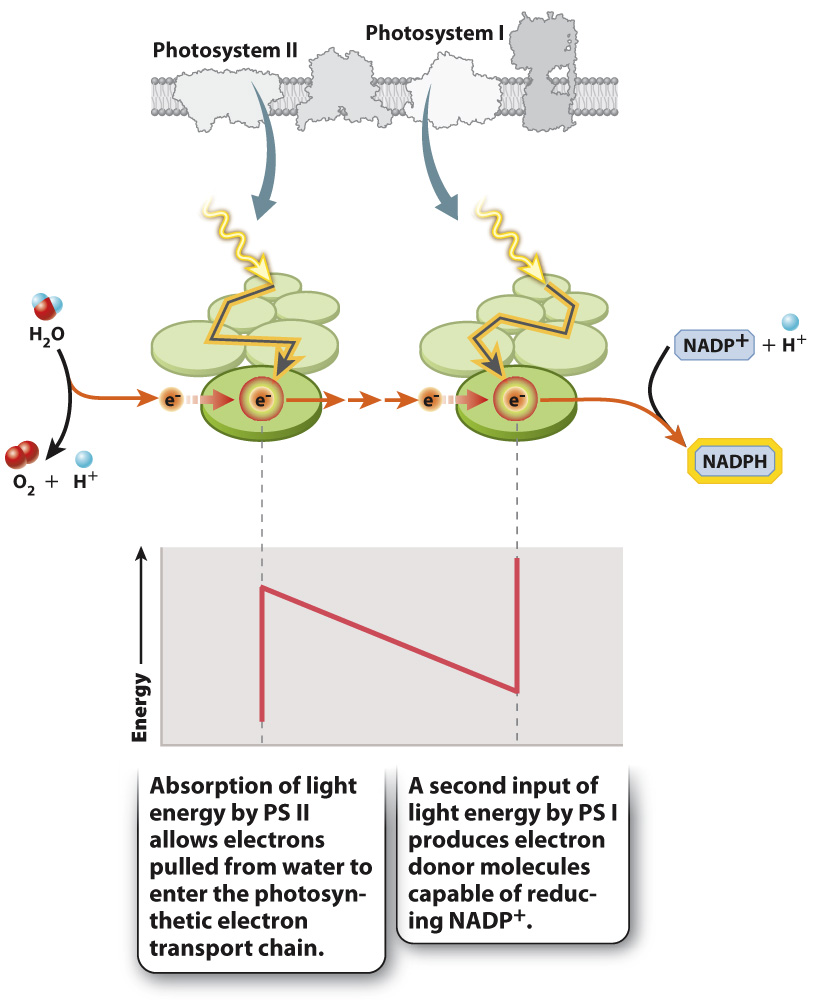
For the two photosystems to work together to move electrons from water to NADPH, they must have distinct chemical properties. Photosystem II supplies electrons to the beginning of the electron transport chain. When photosystem II loses an electron (that is, when it is itself oxidized), it is able to pull electrons from water. In contrast, photosystem I energizes electrons with a second input of light energy so they can be used to reduce NADP+. The key point here is that photosystem I when oxidized is not a sufficiently strong oxidant to split water, whereas photosystem II is not a strong enough reductant to form NADPH.
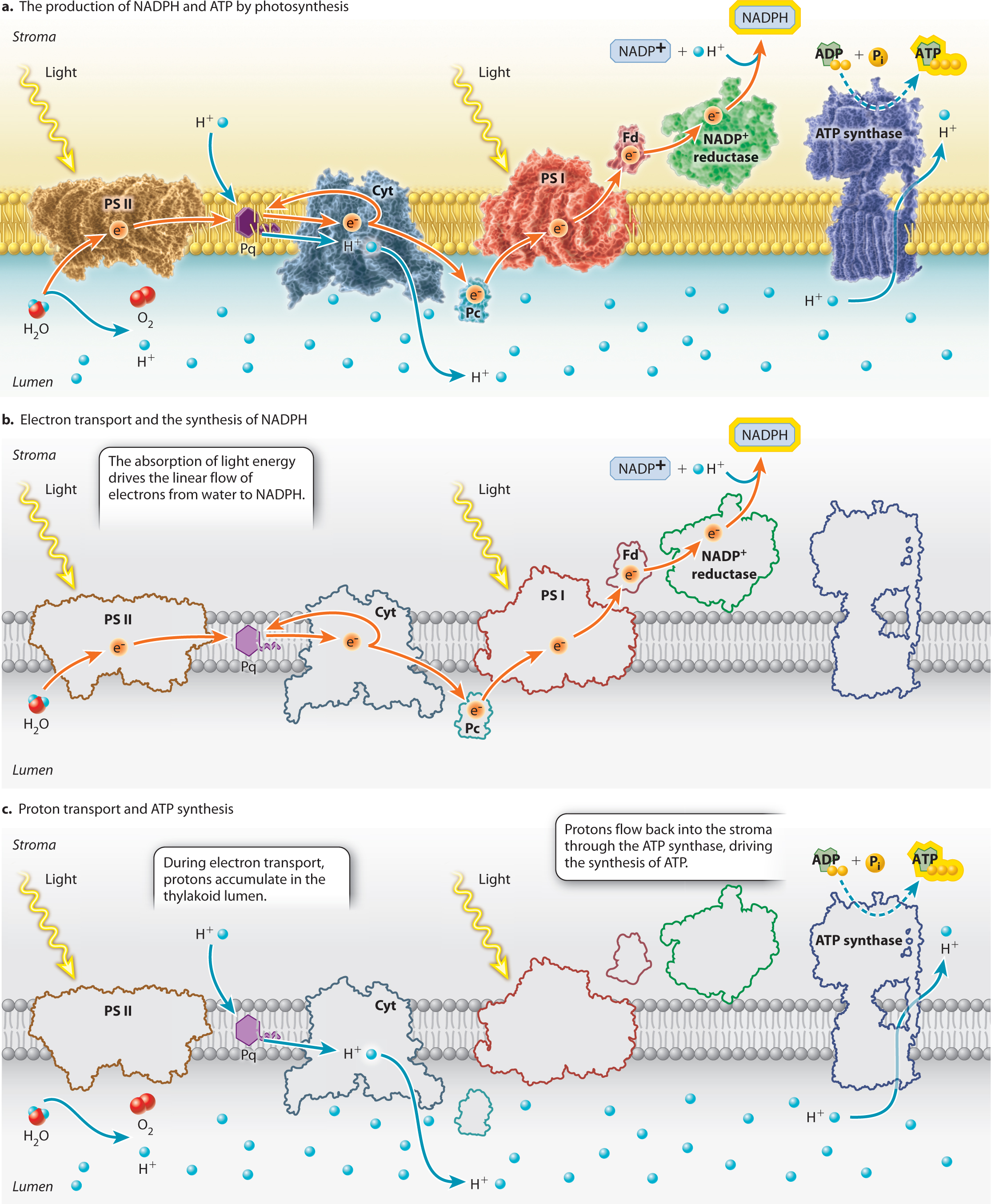
The major protein complexes of the photosynthetic electron transport chain include the two photosystems as well as the cytochrome-
164
Water donates electrons to one end of the photosynthetic electron transport chain, whereas NADP+ accepts electrons at the other end. The enzyme that pulls electrons from water, releasing both H+ and O2, is located on the lumen side of photosystem II. The mechanism by which water splitting occurs is not known, despite the considerable industrial value of developing a way to use sunlight to generate hydrogen gas (H2). NADPH is formed when electrons are passed from photosystem I to a membrane-
NADP+ + 2e− + H+ → NADPH
Quick Check 4 Why are two photosystems needed if H2O is used as an electron donor?
Quick Check 4 Answer
One photosystem is needed to pull electrons from water, and a second photosystem is needed to raise the energy of these electrons enough that they can reduce NADP+.