Cell-surface receptors act like molecular switches.
As we saw earlier, a receptor is activated after a signaling molecule binds to its ligand-binding site. Many receptors act as binary molecular switches, existing in two alternative states, either “on” or “off” (Fig. 9.7). In this way, receptors behave similarly to a light switch (Fig. 9.7a). When bound to their signaling molecule, the molecular switch is turned on. When the signaling molecule is no longer bound, the switch is turned off.
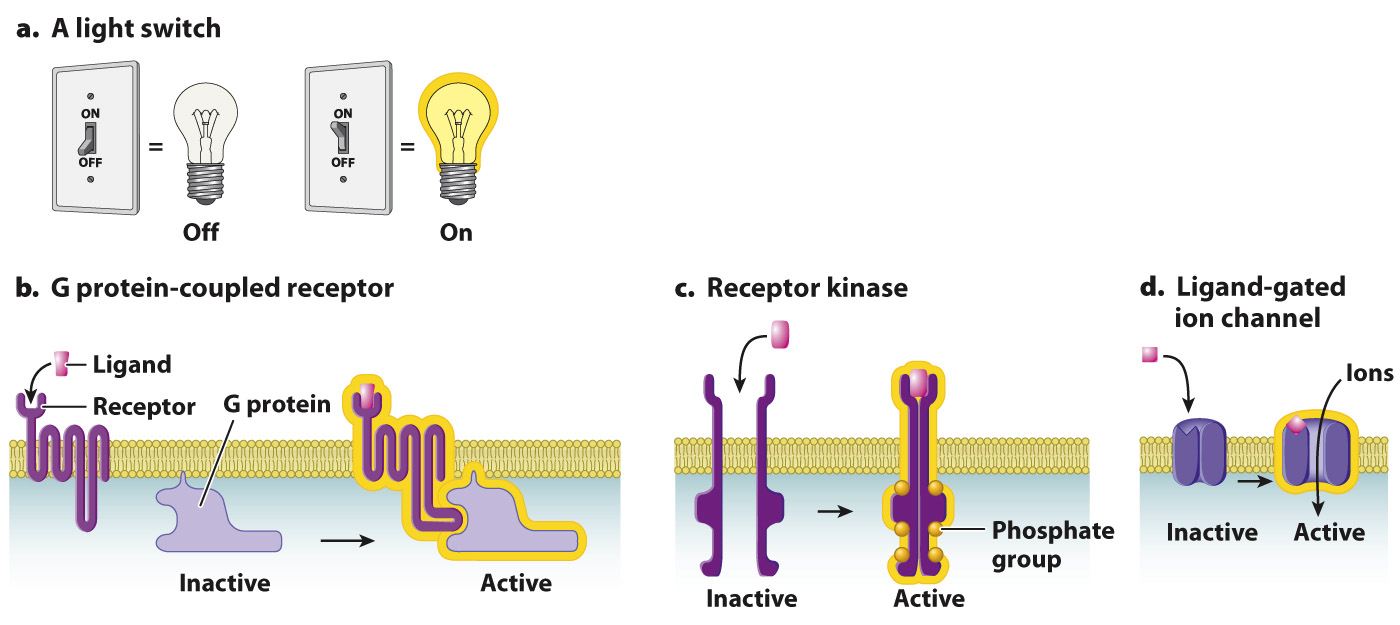
FIG. 9.7 Three types of cell-surface receptor. Receptors act like (a) a light switch. (b) G protein-coupled receptors, (c) receptor kinases, and (d) ligand-gated ion channels can be either “off” (inactive) or “on” (active).
There are thousands of different receptor proteins on the surface of any given cell. Most of them can be placed into one of three groups on the basis of their structures and what occurs immediately after the receptor binds its ligand. One type of cell-surface receptor is called a G protein-coupled receptor (Fig. 9.7b; section 9.4). When a ligand binds to a G protein-coupled receptor, the receptor couples to, or associates with, a G protein, as its name suggests. G protein-coupled receptors are evolutionary conserved and all have a similar molecular structure (section 9.4)
A second group of cell-surface receptors are themselves enzymes, which are activated when the receptor binds its ligand. Most of these are receptor kinases (Fig. 9.7c and section 9.5). A kinase is an enzyme that catalyzes the transfer of a phosphate group from ATP to a substrate. To catalyze this reaction, it binds both ATP and the substrate. This process is called phosphorylation (Fig. 9.8). Phosphorylation is important because it affects the activity of the substrate: When a protein is phosphorylated by a kinase, it typically becomes active and is switched on. The addition of a phosphate group to a protein can activate it by altering its shape or providing a new site for other proteins to bind. Phosphatases remove a phosphate group, a process called dephosphorylation (Fig. 9.8). When a protein is dephosphorylated by a phosphatase, it typically becomes inactive and is switched off.
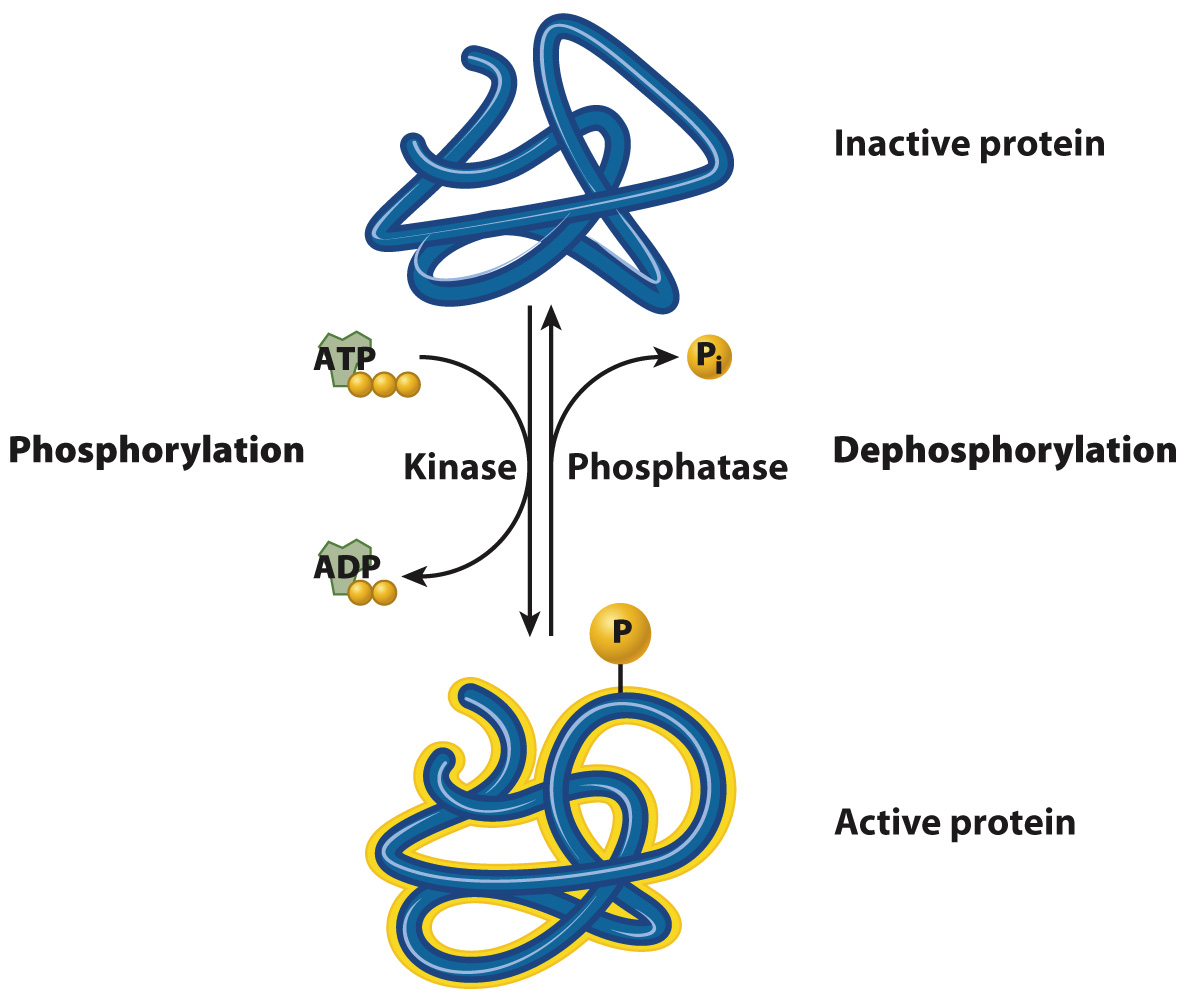
FIG. 9.8 Phosphorylation and dephosphorylation. A kinase transfers a phosphate group from ATP to a protein, typically activating the protein. A phosphatase removes a phosphate group from a protein, typically deactivating the protein.
Receptors in the third group, ion channels, alter the flow of ions across the plasma membrane. These channels can be opened in different ways. Some open in response to changes in voltage across the membrane; these are called voltage-gated ion channels and are discussed in Chapter 35. Other ion channels open when bound by their ligand; these are called ligand-gated ion channels (Fig. 9.7d) and are discussed in Chapter 37. Recall from Chapter 5 that channel proteins help ions and other molecules diffuse into and out of the cell by providing a hydrophilic pathway through the hydrophobic core of the plasma membrane. Most of the time, the channels are closed. However, when a signaling molecule binds to the extracellular portion of a ligand-gated ion channel, the channel undergoes a conformational change that opens it and allows ions to flow in and out. This type of signaling is especially important for nerve and muscle cells since their functions depend on a rapid change in ion flow across the plasma membrane.
What happens after a signaling molecule binds to its receptor and flips a molecular switch? Following receptor activation, signaling pathways transmit the signal to targets in the interior of the cell, the cell responds, and eventually the signal is terminated. In the next two sections, we examine the signaling pathways activated by G protein-coupled receptors and receptor kinases.

