Receptor kinases phosphorylate each other, activate intracellular signaling pathways, lead to a response, and are terminated.
Signaling through receptor kinases follows the same basic sequence of events that we saw in signaling though G protein-coupled receptors, including receptor activation, signal transduction, cellular response, and termination. Let’s consider an example. Think about the last time you got a cut. The cut likely bled for a minute or two, and then the bleeding stopped. The signaling molecule platelet-derived growth factor (PDGF) helps the healing process get started. When platelets in the blood encounter damaged tissue, they release a number of proteins, including PDGF. PDGF is the signaling molecule that binds to PDGF-specific receptor kinases on the surface of cells at the site of a wound.
Receptor kinases have an extracellular portion that binds the signaling molecule and an intracellular portion that is a kinase, an enzyme that transfers a phosphate group from ATP to another molecule. A single molecule of PDGF binds to the extracellular portion of two receptors, causing the receptors to partner with each other. This partnering of two similar or identical molecules is called dimerization. Dimerization activates the cytoplasmic kinase domains of the paired receptors, causing them to phosphorylate each other at multiple sites on their cytoplasmic tails (Fig. 9.14). The addition of these phosphate groups provides places on the receptor where other proteins bind and become active.
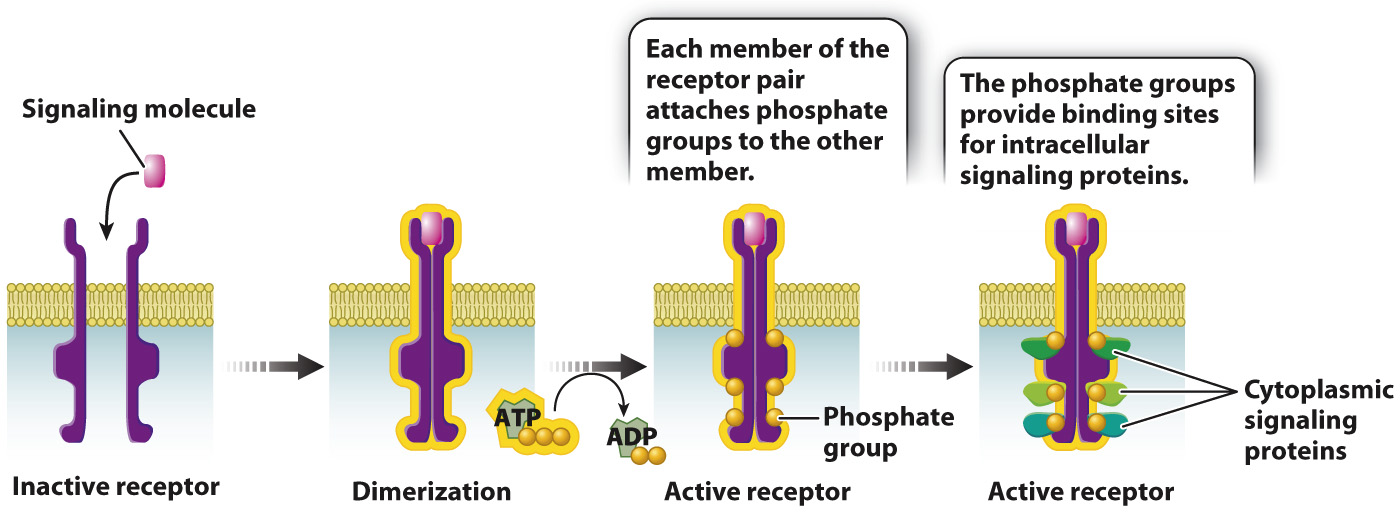
FIG. 9.14 Receptor kinase activation and signaling. Receptor kinases bind signaling molecules, dimerize, phosphorylate each other, and activate intracellular signal molecules.
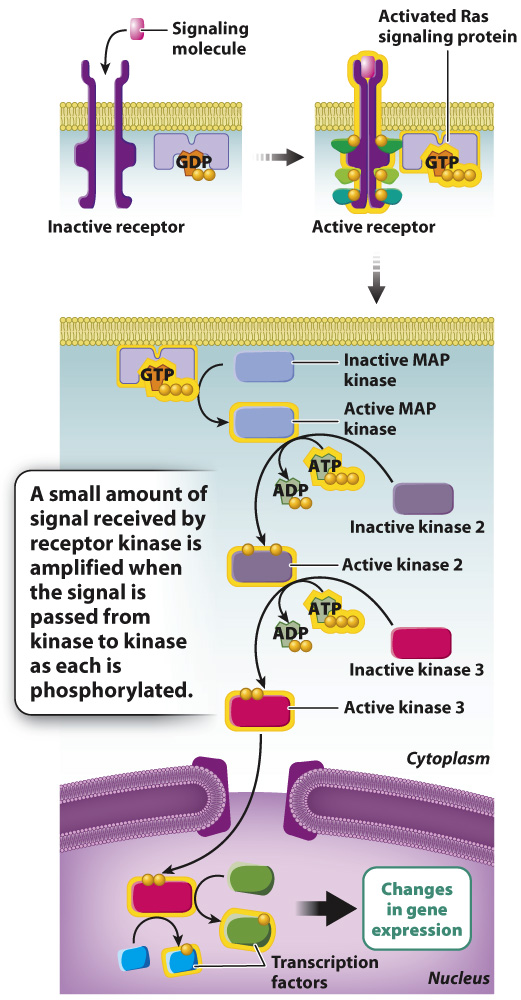
FIG. 9.15 The MAP kinase pathway. Some receptor kinases signal through Ras, which in turn activates the MAP kinase pathway, leading to changes in gene expression.
One of the downstream targets of an activated receptor kinase is Ras. Ras is a G protein, but in contrast to the three-subunit G proteins discussed earlier, Ras consists of a single subunit, similar to the α subunit of the three-subunit G proteins. In the absence of a signal, Ras is bound to GDP and is inactive. However, when Ras is activated by a receptor kinase, it exchanges GDP for GTP. Activated GTP-bound Ras triggers the activation of a protein kinase that is the first in a series of kinases that are activated in turn, as each kinase phosphorylates the next in the series. The series of kinases collectively are called the mitogen-activated protein kinase pathway, or MAP kinase pathway (Fig. 9.15). The final activated kinase in the series enters the nucleus, where it phosphorylates target proteins. Some of these proteins include transcription factors that turn on genes needed for cell division so that your cut can heal.
The signals received by receptor kinases are amplified as the signal is passed from kinase to kinase. Each phosphorylated kinase in the series activates multiple molecules of the downstream kinase, and the downstream kinase in turn activates many molecules of another kinase still farther downstream. In this way, a very small amount of signaling molecule (PDGF in our example) can cause a large-scale response in the cell.
Receptor kinase signaling is terminated by the same basic mechanisms that are at work in G protein-coupled receptor pathways. For example, protein phosphatases inactivate receptor kinases and other enzymes of the MAP kinase pathway. Furthermore, Ras hydrolyzes GTP to GDP, just like the G protein α subunit. Shortly after Ras binds to GTP and becomes active, Ras converts GTP to GDP and becomes inactive. Without an active receptor kinase to generate more active Ras, activation of the MAP kinase pathway stops.

