2.8–2.11: Carbohydrates are fuel for living machines.

Hardly a day goes by without an item in a magazine or newspaper or on TV talking about whether carbohydrates are good or bad for us. Sports drinks such as Gatorade are filled with carbs, and in the days before a big game or race, athletes often “carbo-
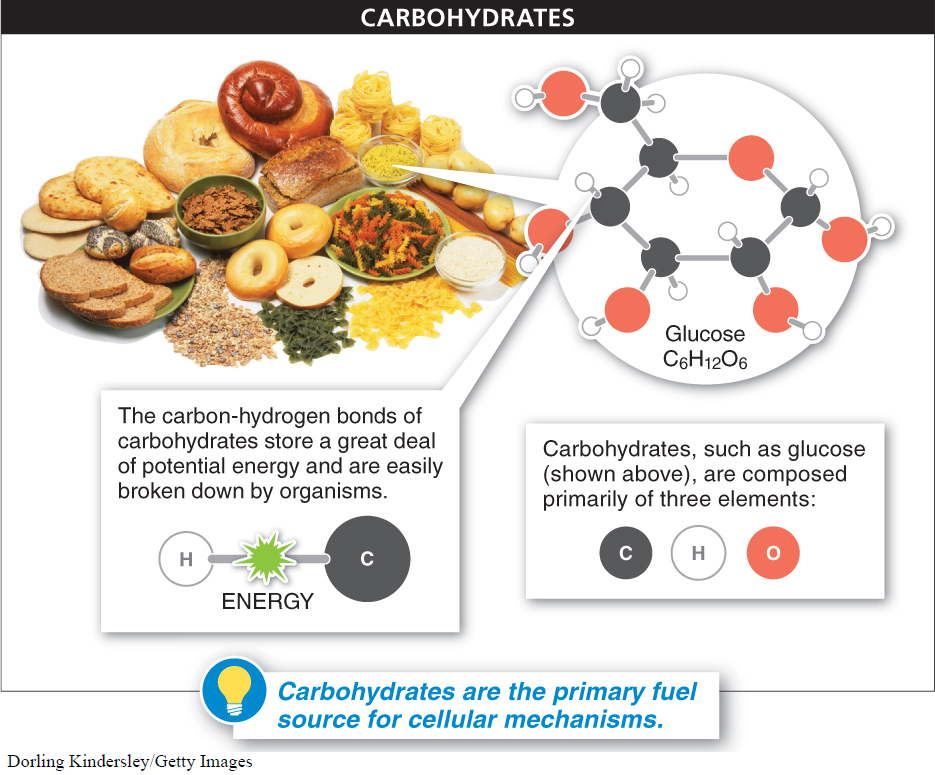
Carbohydrates are one type of macromolecule—a large molecule made up from smaller building blocks or subunits. Four types of macromolecule are essential to the building and functioning of living organisms: carbohydrates, lipids, proteins, and nucleic acids. Carbohydrates are molecules that contain carbon, hydrogen, and oxygen: they are the primary fuel for running all of the cellular machinery and also form much of the structure of cells in all life forms. Sometimes they contain atoms of other elements, but they must have carbon, hydrogen, and oxygen to be considered a carbohydrate (FIGURE 2-20). Additionally, a carbohydrate generally has approximately the same number of carbon atoms as it does H2O units. For instance, the best-
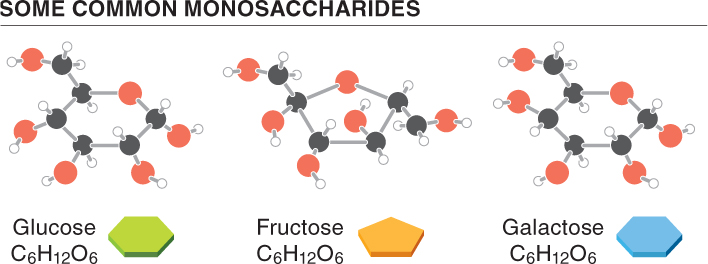
Carbohydrates are classified into several categories, based on their size and their composition. The simplest carbohydrates are the monosaccharides, or simple sugars. The simple sugars contain anywhere from three to six carbon atoms and, when they are broken down, the products usually are not carbohydrates. Two common monosaccharides are glucose, found in the sap and fruit of many plants, and fructose, found primarily in fruits and vegetables, as well as in honey. Fructose is the sweetest of all naturally occurring sugars. The suffix -ose tells us that a substance is a carbohydrate.
56
Carbohydrates function well as fuels because of their many carbon-
In the next section we investigate glucose, the chief carbohydrate used by organisms to fuel their activities.
TAKE-HOME MESSAGE 2.8
Carbohydrates are the primary fuel for running all cellular machinery and also form much of the structure of cells in all life forms. Carbohydrates contain carbon, hydrogen, and oxygen, and generally have the same number of carbon atoms as they do H2O units. The simplest carbohydrates, including glucose, are monosaccharides or simple sugars. They contain from three to six carbon atoms. As the chemical bonds of carbohydrates are broken down and other more stable bonds are formed, a great deal of energy is released that can be used by the organism.
Why do carbohydrate molecules function so well as fuels for the body?