6.1–6.4: There are different types of cell division.
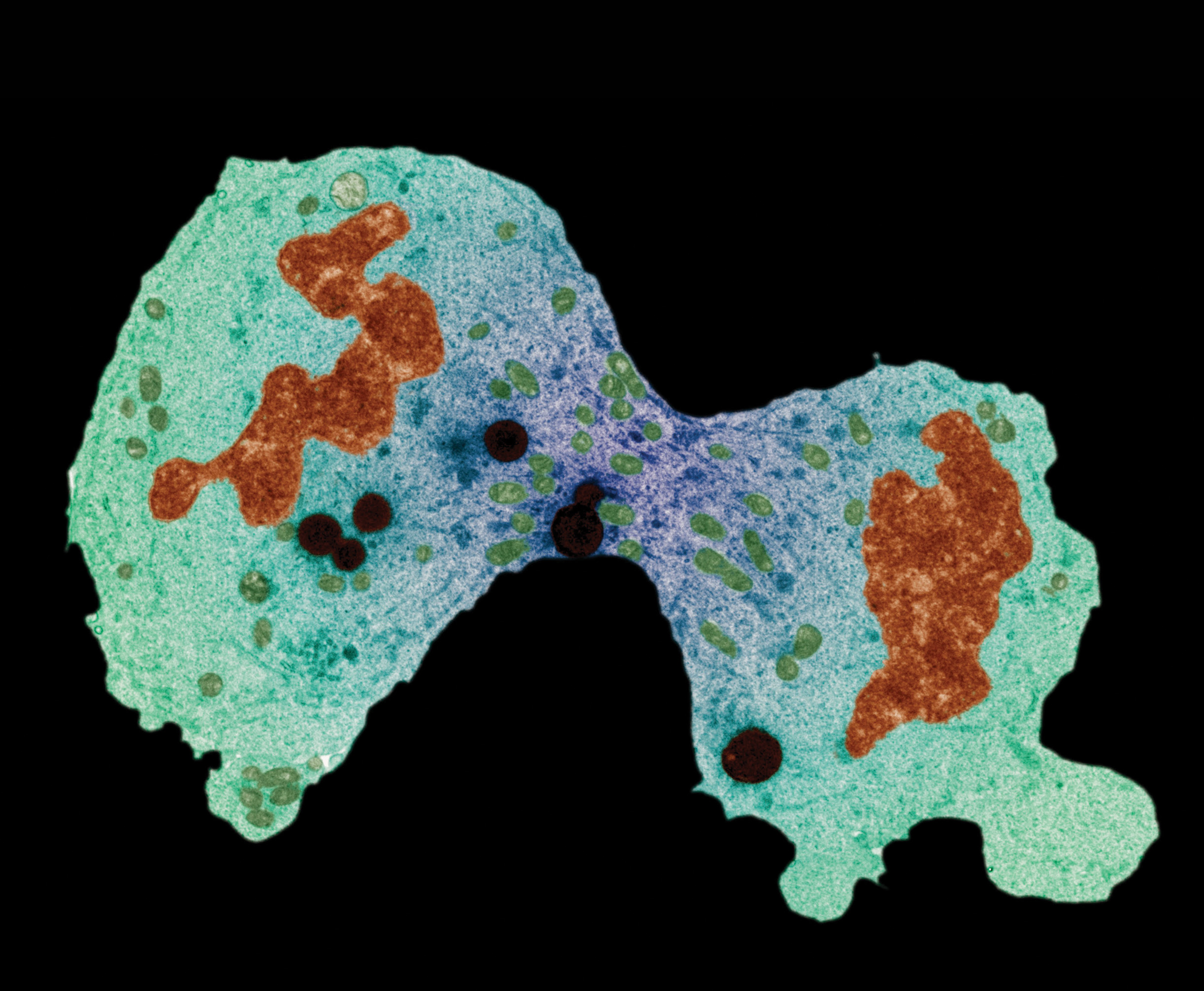
Once you are fully grown, do you have just one complete set of cells that live as long as you do? The answer is no. Although some of the cells in your body may last for many decades, throughout most of your body your cells are continually dying off, and the ones that remain divide and replace the cells you’ve lost, in an ongoing process. But can this cell replacement go on forever? And does a cell even know how old it is?
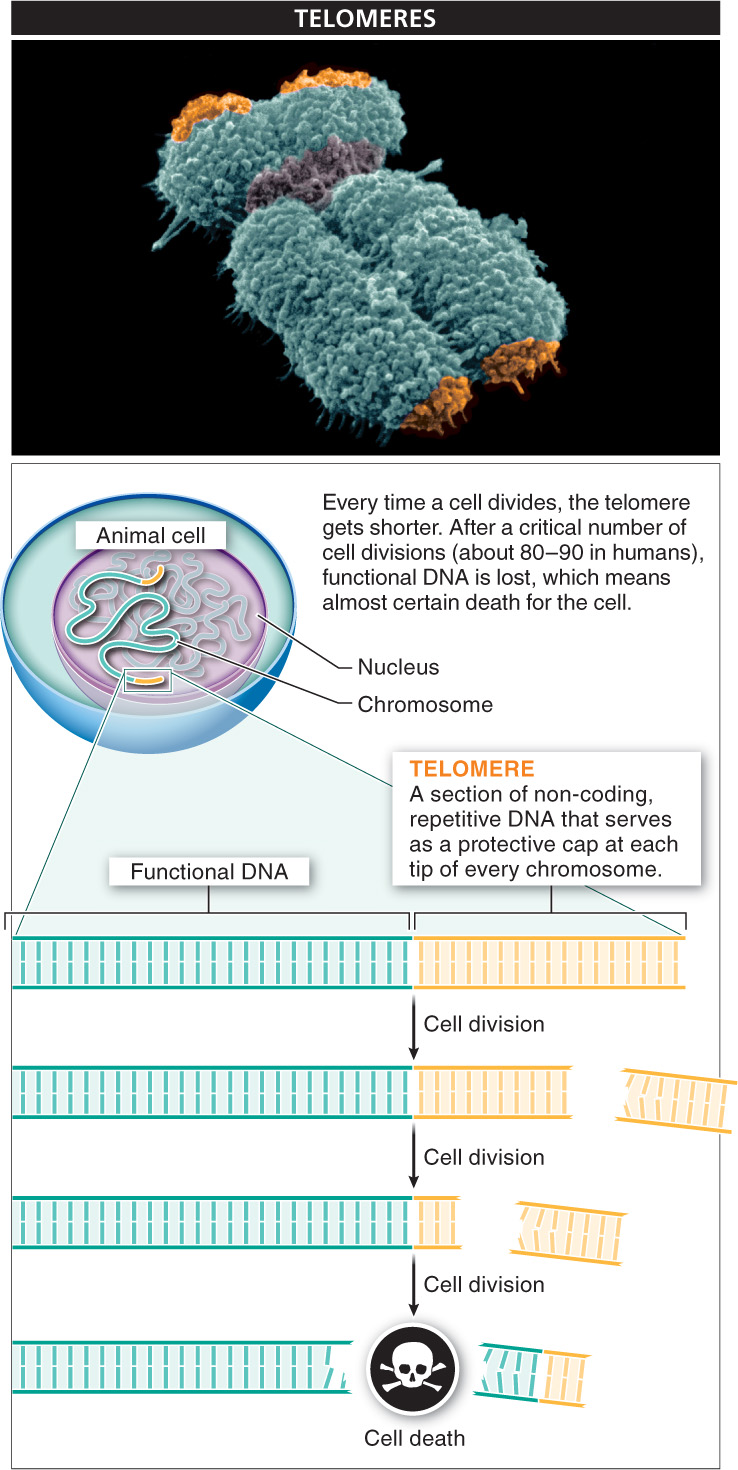
Actually, a cell does have a feature that provides an approximate measure of how old it is. Just as a car comes with an odometer, which keeps track of how far the car has been driven, animal cells have a sort of counter that reflects how many times the cell has divided. This “counter” is a section of non-
Every time a cell divides, making an exact copy of itself, its DNA divides as well. However, each time the DNA divides, the process by which chromosomes are duplicated causes the telomere at each end of every chromosome to get a bit shorter. After many cell divisions, the telomeres can become so short that additional cell divisions cause the loss of functional, essential DNA, and that means almost certain death for the cell.
Telomeres in humans are repeats of the nucleotide sequence TTAGGG. It’s the identical sequence in many vertebrate species and very similar in most other eukaryotes. The number of times the sequence repeats varies across species; in humans, the number is about 2,000. At birth, the telomeres in most human cells are long enough to support 80–
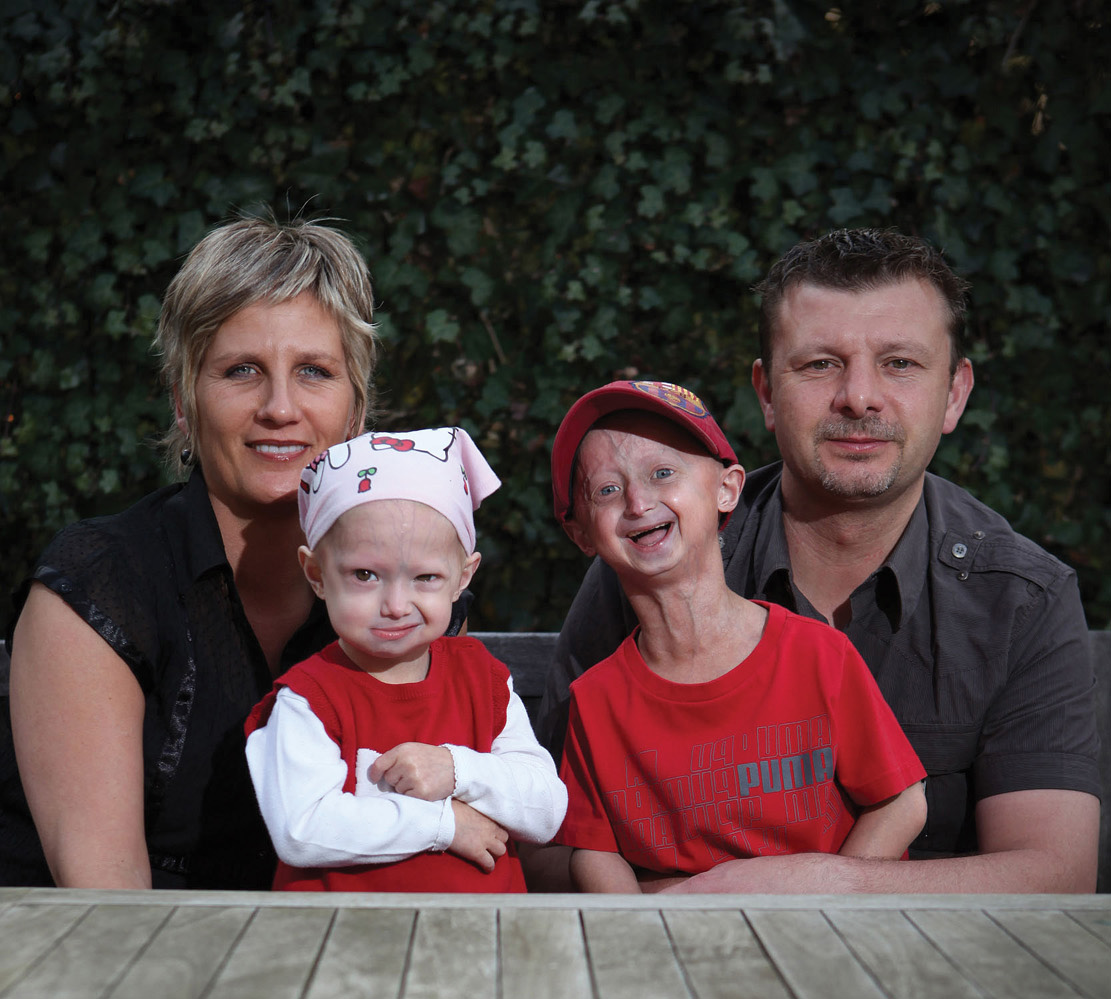
Occasionally, individuals are born with telomeres that impair functioning of a protein that helps maintain the nucleus of cells. This interferes with normal cell functioning in numerous ways, including damaging the cell’s telomeres, causing them to be much shorter than normal. In these people, the normal functioning of many genes is disrupted, and their cells and tissues begin to appear aged very soon after birth (FIGURE 6-2). As a consequence, children born with this disorder rarely live beyond the age of 13.
This observation, in conjunction with the observation that telomeres get shorter with each cell division, might seem to suggest that continually rebuilding the telomere after each round of division would be an effective strategy for allowing a cell and its descendants to function for a longer time than normal. Such a line of cells would never die. By extension, it’s tempting to imagine that constantly rebuilding telomeres might act like a fountain of youth. Unfortunately, it does not.
We know this because there are some cells that do acquire this feature. These cells rebuild their telomeres after each cell division, restoring the chromosomes’ protective caps. For single-
3
Unfortunately, for most of the other types of cells that rebuild their telomeres with each cell division, the telomere rebuilding presents a big problem: the cells are unable to stop dividing. Such cells commonly go by another name: cancer. Because telomere rebuilding occurs in (and possibly is necessary to) many human cancers, researchers have hopes that inhibiting it might help fight cancer. This process is already the target of some anti-
In this chapter, we investigate the processes that enable cells to divide and create new cells. Prokaryotes exhibit one method of cell division (called binary fission), and it serves all of their cell division needs. Eukaryotes exhibit two methods of cell division, mitosis and meiosis, each of which has a specific purpose in a eukaryote’s life cycle. We’ll explore normal cell division and discuss what happens when cell division does not proceed in the normal way.
TAKE-HOME MESSAGE 6.1
Cell division is an ongoing process in most organisms and their tissues; disruptions to normal cell division can have serious consequences. In eukaryotic cells, a protective section of DNA called the telomere, at each end of every chromosome, plays a role in keeping track of cell division, getting shorter every time the cell divides. If telomeres become too short, additional cell divisions cause the loss of essential DNA and cell death. Cells that rebuild their telomeres with each division can become cancerous.
Where on a chromosome are telomeres, and how are they involved in limiting the number of times a cell can divide?
4