
Chapter 4. Chapter 4: Energy
Review & Rehearse

Instructions
Review the visual summaries and answer the essay questions below.
Make sure to enter a brief response that completely answers each question and explains your reasoning. When you click "Submit," you will be provided instant feedback, allowing you to check if your response is correct.
(This activity contains 17 total essay questions. Each new question will be revealed once you complete the preceding question.)
1.

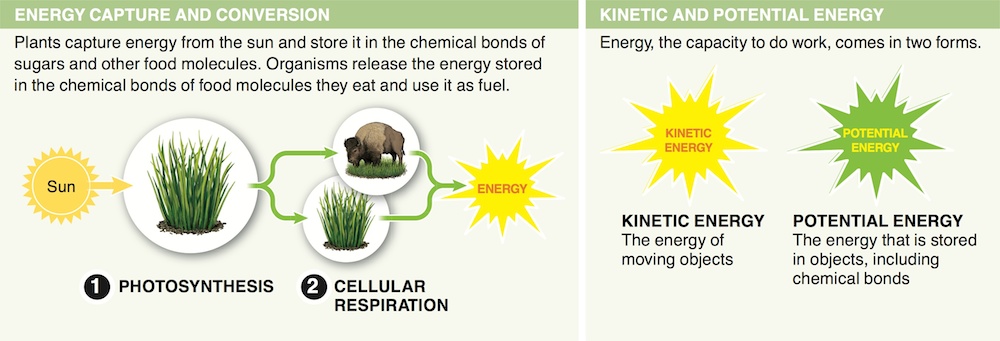
1. Why are fossil fuels considered a non-renewable resource?
2.
2. In terms of energy, describe what happens when a ball at the top of a steep ramp is released.
3.
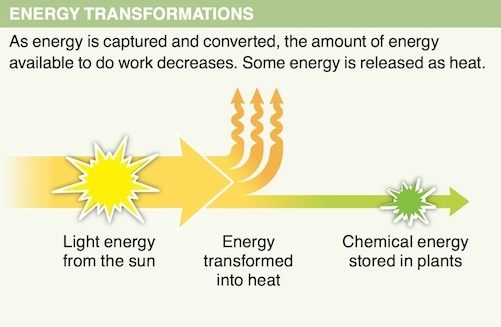
3. Why is the conversion of energy from one form to another considered inefficient?
4.
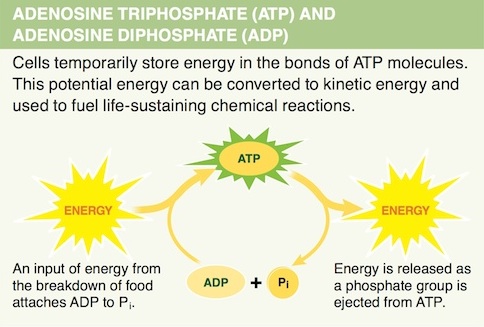
4. Which chemical feature of ATP makes it effective in carrying and storing energy?
5.

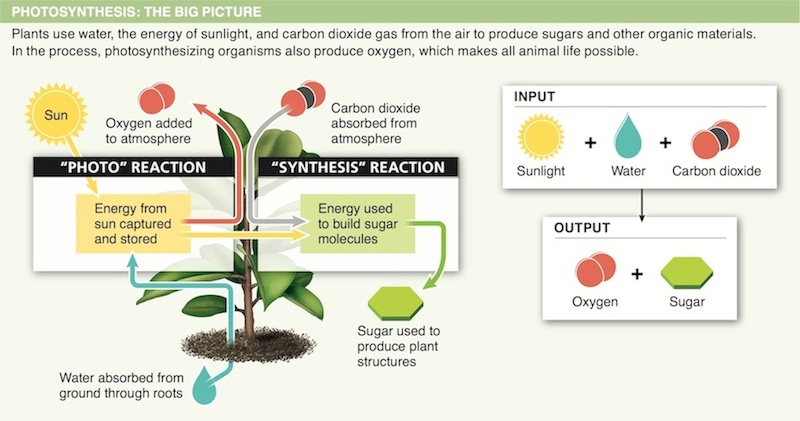
5. Which by-product of photosynthesis is necessary for most animals to live?
6.
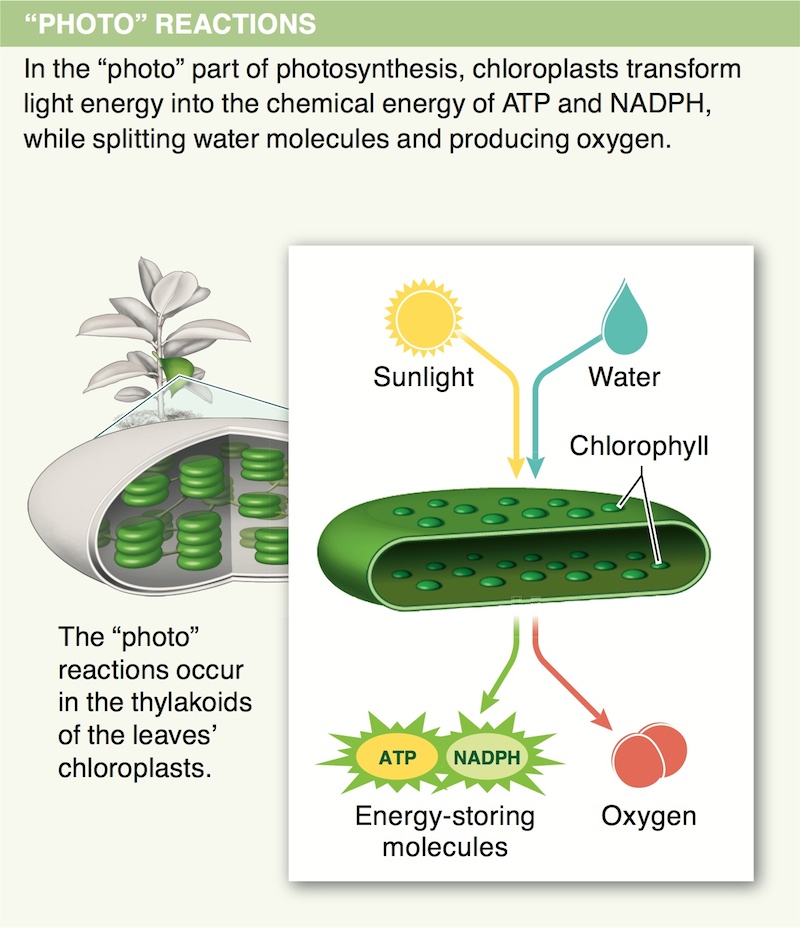
6. In which parts of a plant are most of its chloroplasts typically found? Why?
7.
7. Why do leaves usually appear green?
8.
8. Describe one of the chief ways in which energy “moves” through cells within a plant.
9.
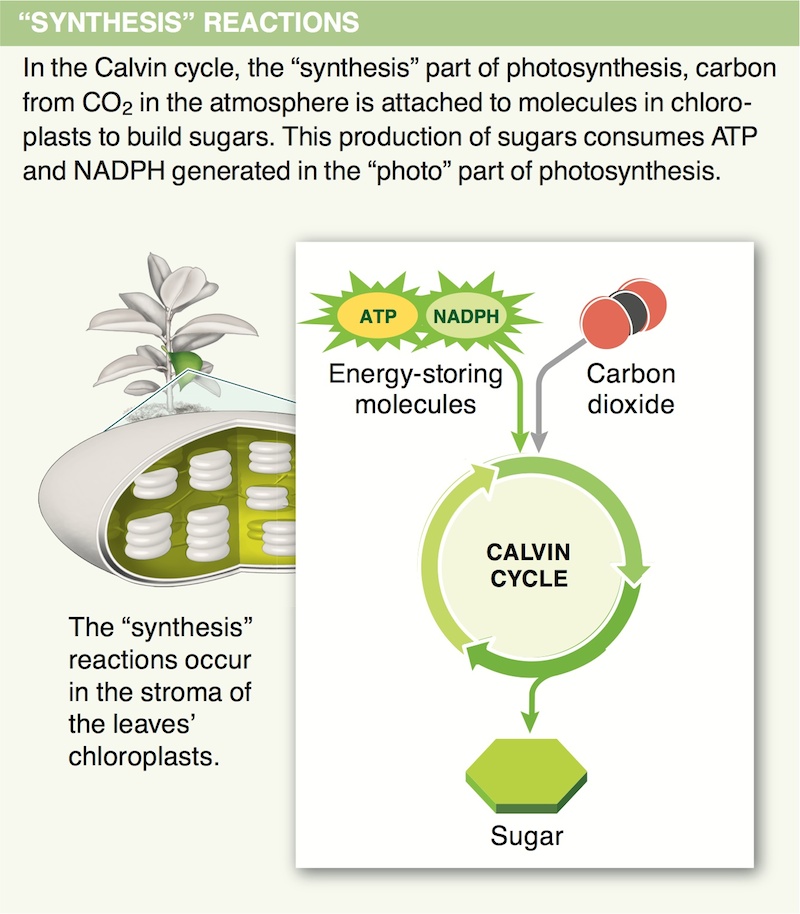
9. As they are excited by light energy during photosynthesis, electrons sometimes move from one molecule to another. Other times they do not. Explain this difference.
10.
10. Rubisco is the most abundant protein on earth. What does it do?
11.
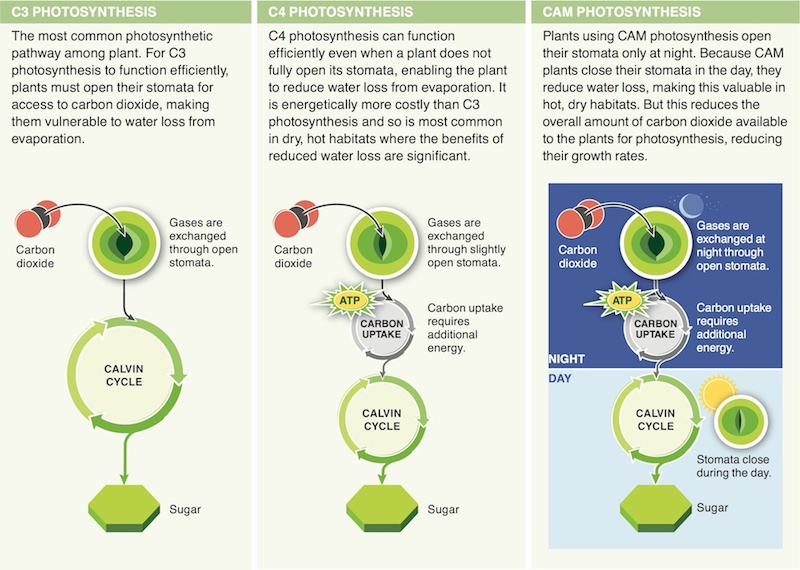
11. To reduce water loss via evaporation, plants may close their stomata. Although this action solves one problem for a plant, it creates another. Describe the new problem.
12.

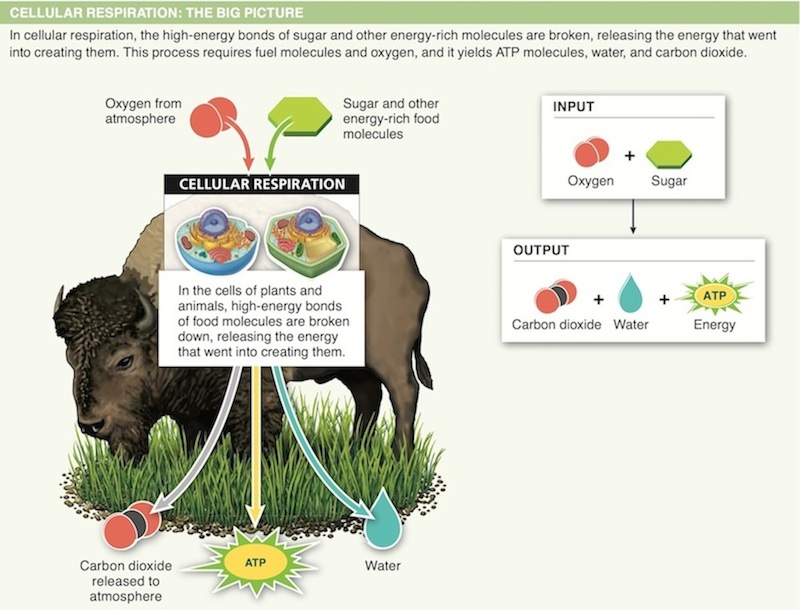
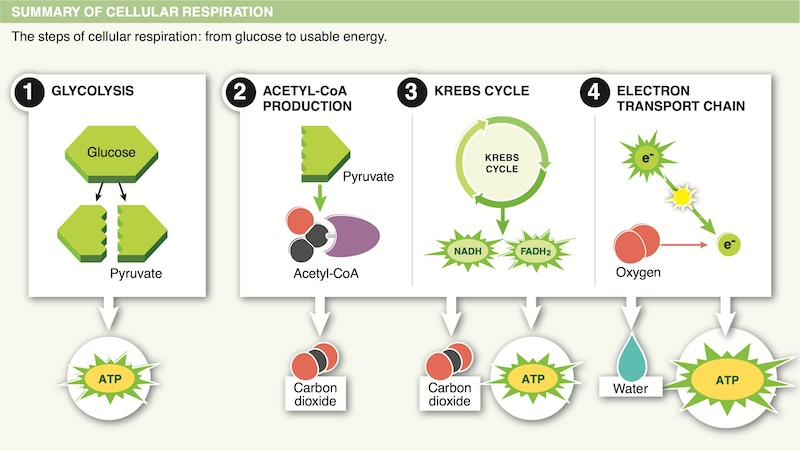
12. Organisms harness energy from “food” molecules during cellular respiration. Where in the food does the energy come from?
13.
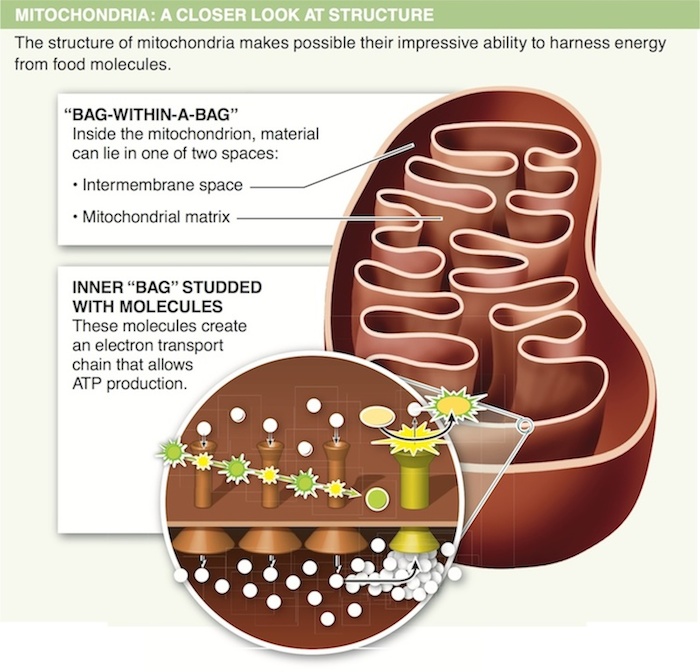
13. In which organelle does glycolysis occur?
14.
14. Why are the mitochondria considered to be ATP factories?
15.
15. Describe how a structural feature of mitochondria helps them in the production of ATP.
16.

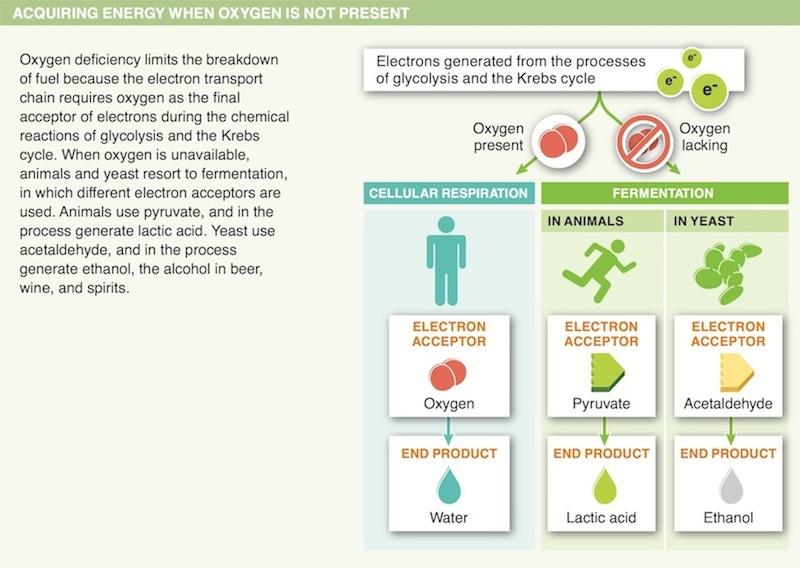
16. A lack of oxygen limits the breakdown of fuel for most animals. Why?
17.
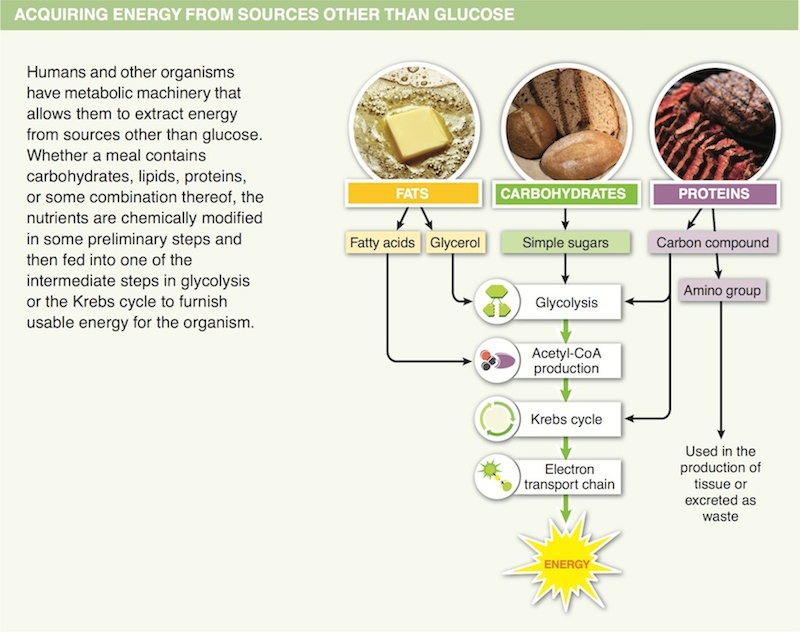
17. In the initial breakdown of dietary lipids, what molecules are formed?
Activity results are being submitted...