26.6 The structure of antibodies reflects their function.
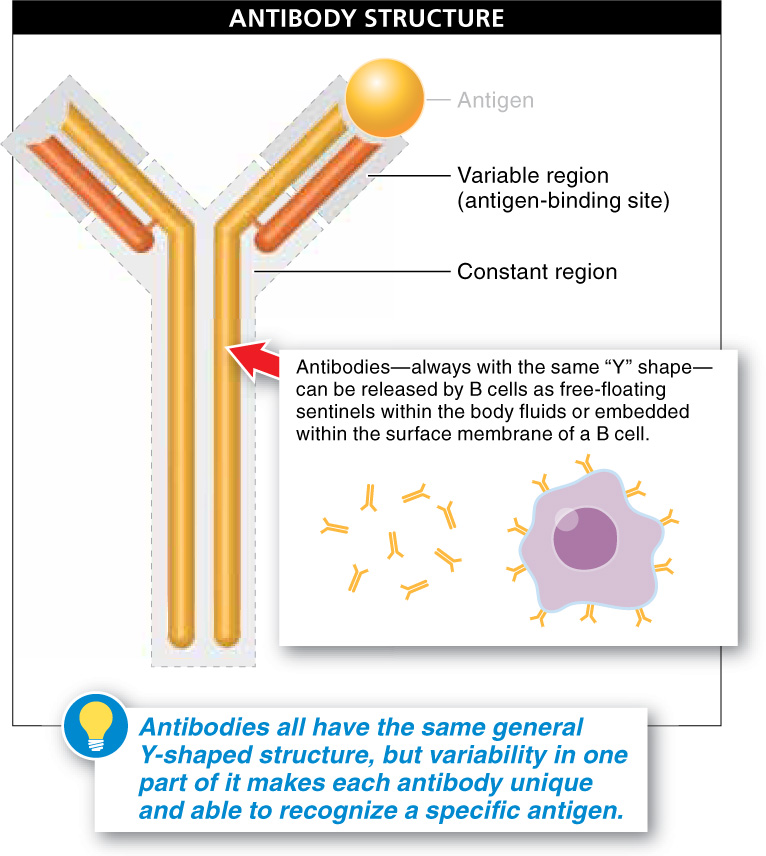
Figure 26.20: Variations on a molecular theme. The shape at the top of the “Y” varies from one antibody to another and fits with only one antigen.
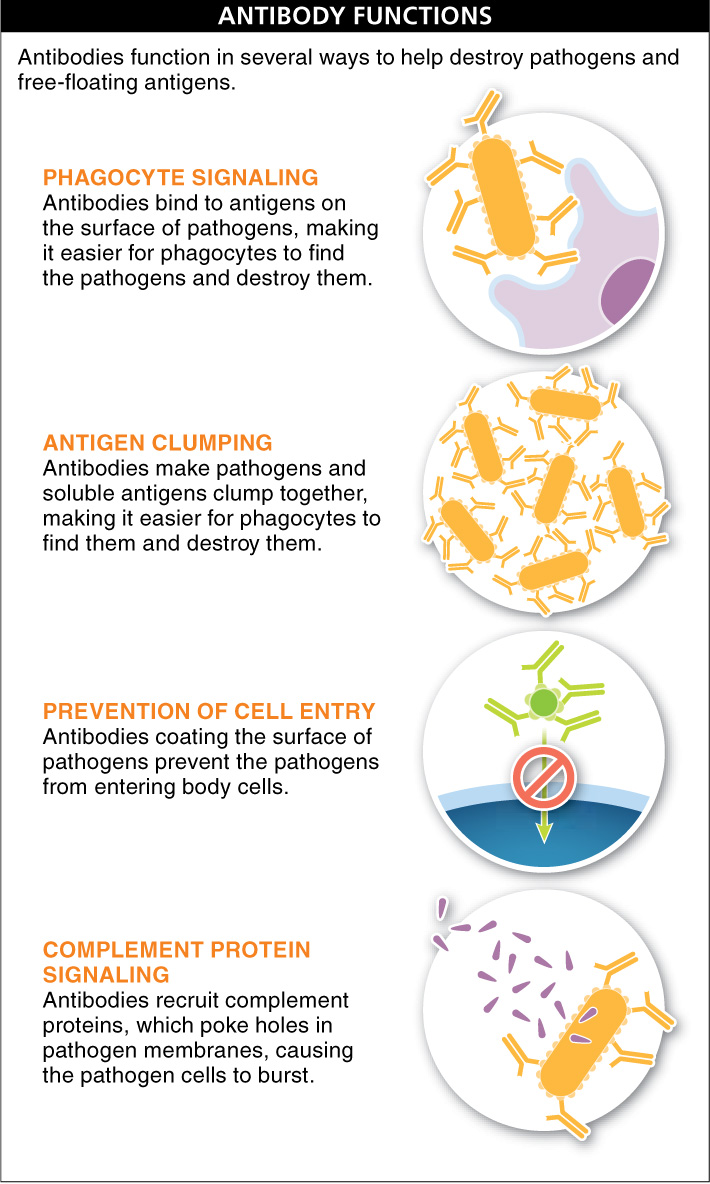
Figure 26.21: Antibodies fight pathogens in several ways.
We now examine the structure and function of antibodies to learn exactly how they protect us against pathogens. All antibodies are Y-shaped, and their diversity and specificity come from variations in the top of the “Y.”
Each antibody consists of four polypeptide chains joined together: two long (also called heavy) chains and two short (or light) chains (FIGURE 26-20). The top of the “Y” is the region that varies from antibody to antibody. But there are only about five variants for the lower part, the base of the “Y,” and so this is referred to as the constant region of an antibody. At the top of the “Y,” the variable region, each antibody has a unique three-dimensional shape that dictates which specific antigen it will “fit.” An enormous diversity of antibodies is needed to recognize the correspondingly enormous group of antigens that the immune system may encounter. (And a given pathogen may have several different types of antigens on its surface.) Keep in mind that antibodies recognize antigens, not pathogens. An antigen is anything that an antibody recognizes as not being part of the body, as not being “self,” including snake venom toxins, bacterial toxins that cause food poisoning, pollen, and various chemicals (such as those found in latex gloves).
Any foreign cells introduced into the body will elicit an immune response, even if the cells are part of a life-saving medical treatment. Consider organ transplants. Transplanted cells have surface molecules that are seen as antigens (“non-self”) by the recipient’s immune system. Although the transplanted cells are not pathogens, the recipient’s immune system identifies them as invaders, and an immune response begins. Thus, medicines are needed to suppress the immune system’s attack on and eventual destruction of a transplanted organ.
The immune system is also the reason that people in need of blood transfusions must have a proper match of blood type. As we saw in Section 7-10, if a transfusion is made between individuals with incompatible blood types, the recipient’s antibodies will attach to antigens on the surface of the foreign (in this case, the donor’s) red blood cells and mount an attack that can end in death.
Now let’s consider the main ways in which antibodies function (FIGURE 26-21). The immune system pumps out tons of antibodies following exposure to a pathogen, and these antibodies attach to antigens. When the antigens on the surface of a pathogen have an antibody attached to them, the pathogen is easily ingested by phagocytes (such as macrophages and neutrophils) of the non-specific immune system.
Besides “marking” for destruction the antigens on a pathogen, or marking free-floating antigens (such as snake venom toxin), antibodies act in two other important ways. (1) They cause pathogens or antigens to clump together, making it easier for phagocytes to find and ingest them. And (2) they coat the surfaces of pathogens and prevent them from binding to and entering body cells, and thus they block the infection from spreading.
Lastly, when antibodies are bound to antigens on the surface of a pathogen, they recruit another player of the non-specific system: the complement proteins. Recall that some complement proteins can poke holes in membranes and cause the pathogenic cells to burst. Thus, by recruiting these complement proteins, antibodies indirectly destroy pathogens.
TAKE-HOME MESSAGE 26.6
Each antibody has a unique structure that recognizes a specific antigen. Antibodies are effective in helping to destroy pathogens and free-floating antigens in several ways: by enhancing ingestion by phagocytes, preventing the infection of more cells, and enhancing the destruction of pathogens by complement proteins.
Describe how antibodies help to destroy pathogens.

