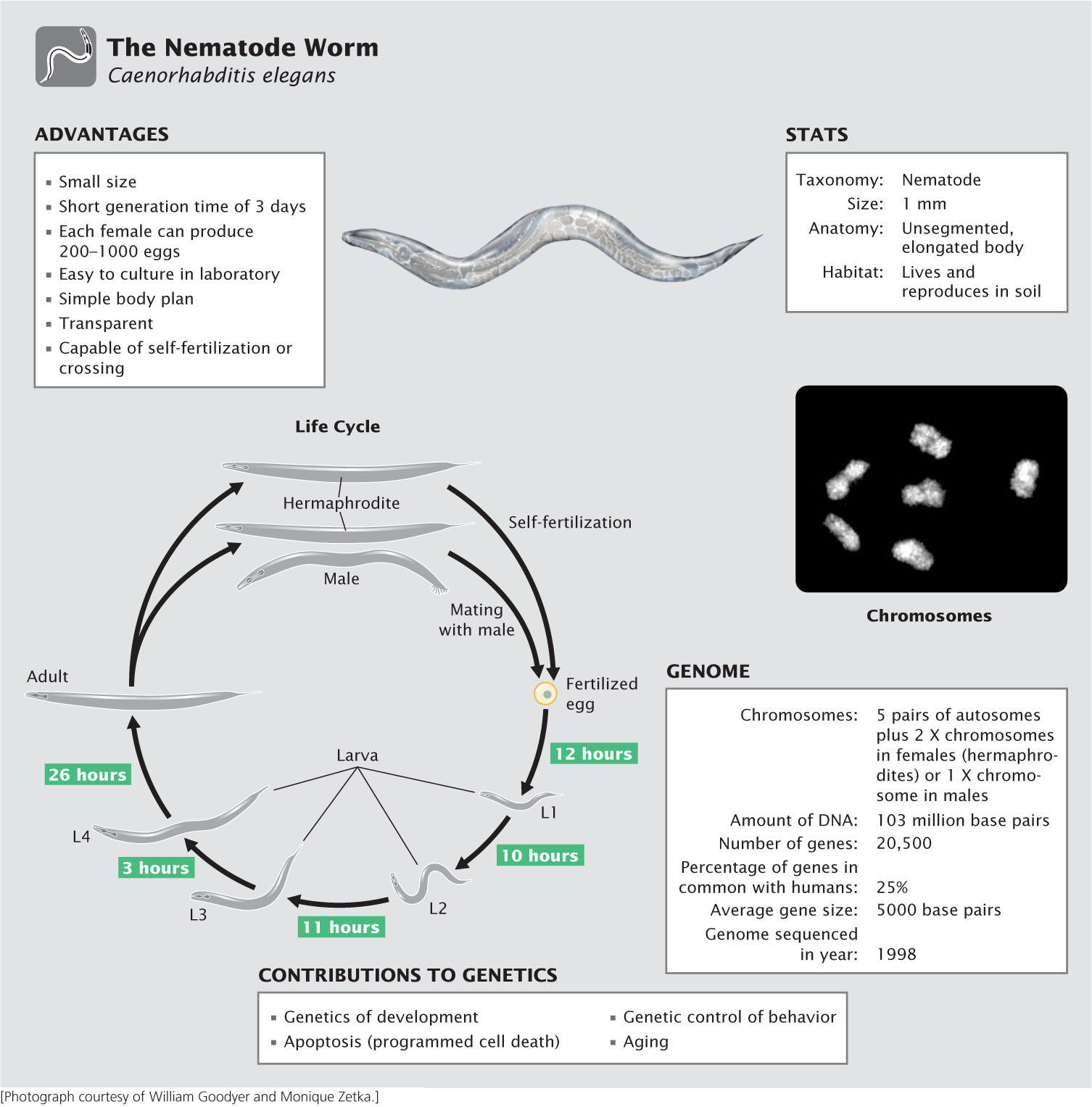
The Nematode Worm Caenorhabditis elegans
You may be asking, What is a nematode, and why is it a model genetic organism? Although rarely seen, nematodes are among the most abundant organisms on Earth, inhabiting soils throughout the world. Most are free living and cause no harm, but a few are important parasites of plants and animals, including humans. Although Caenorhabditis elegans has no economic or medical importance, it has become widely used in genetic studies because of its simple body plan, ease of culture, and high reproductive capacity. First introduced to the study of genetics by Sydney Brenner, who formulated plans in 1962 to use C. elegans for the genetic dissection of behavior, this species has made important contributions to the study of development, cell death, aging, and behavior.
Advantages of C. elegans as a model genetic organism
An ideal genetic organism, C. elegans is small, easy to culture, and produces large numbers of offspring. The adult C. elegans is about 1 mm in length. Most investigators grow C. elegans on agar-filled petri plates that are covered with a lawn of bacteria, which the nematodes devour. Thousands of worms can be easily cultured in a single laboratory. Compared with most multicellular animals, they have a very short generation time, about 3 days at room temperature. And they are prolific reproducers, with a single female producing from 250 to 1000 fertilized eggs in 3 to 4 days.
Another advantage of C. elegans, particularly for developmental studies, is that the worm is transparent, allowing easy observation of internal development at all stages. It has a simple body structure, with a small, invariant number of somatic cells: 959 cells in a mature hermaphroditic female and 1031 cells in a mature male.
Life cycle of C. elegans
Most mature adults are hermaphrodites, with the ability to produce both eggs and sperm and undergo self-fertilization. A few are male, which produce only sperm and mate with hermaphrodites. The hermaphrodites have two sex chromosomes (XX); the males possess a single sex chromosome (XO). Thus, hermaphrodites that self-fertilize produce only hermaphrodites (with the exception of a few males that result from nondisjunction of the X chromosomes). When hermaphrodites mate with males, half of the progeny are XX hermaphrodites and half are XO males.
Eggs are fertilized internally, either from sperm produced by the hermaphrodite or from sperm contributed by a male. The eggs are then laid, and development is completed externally. Approximately 14 hours after fertilization, a larva hatches from the egg and goes through four larval stages—termed L1, L2, L3, and L4—that are separated by molts. The L4 larva undergoes a final molt to produce the adult worm. Under normal laboratory conditions, worms will live for 2 to 3 weeks.
The C. elegans genome
Geneticists began developing plans in 1989 to sequence the genome of C. elegans, and the complete genome sequence was obtained in 1998. Compared with the genomes of most multicellular animals, that of C. elegans, at 103 million base pairs of DNA, is small, which facilitates genomic analysis. The availability of the complete genome sequence provides a great deal of information about gene structure, function, and organization in this species. For example, the process of programmed cell death (apoptosis, see Chapter 22) plays an important role in development and in the suppression of cancer. Apoptosis in C. elegans is remarkably similar to that in humans. Having the complete genome sequence of C. elegans, and given its ease of genetic manipulation, geneticists have identified genes that participate in apoptosis, which has increased our understanding of apoptosis in humans and its role in cancer.
Genetic techniques with C. elegans
Chemical mutagens are routinely used to generate mutations in C. elegans—mutations that are easy to identify and isolate. The ability of hermaphrodites to self-fertilize means that progeny homozygous for recessive mutations can be obtained in a single generation; the existence of males means that genetic crosses can be carried out.
Developmental studies are facilitated by the transparent body of the worms. As stated earlier, C. elegans has a small and exact number of somatic cells. Researchers studying the development of C. elegans have meticulously mapped the entire cell lineage of the species, and so the developmental fate of every cell in the adult body can be traced to the original single-celled fertilized egg. Developmental biologists often use lasers to destroy (ablate) specific cells in a developing worm and then study the effects on physiology, development, and behavior.
829
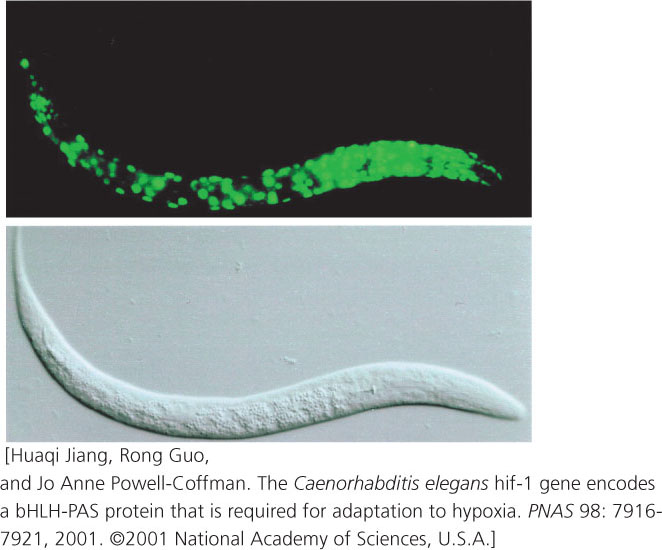
RNA interference has proved to be an effective tool for turning off genes in C. elegans. Geneticists inject double-stranded copies of RNA that is complementary to specific genes; the double-stranded RNA then silences the expression of these genes through the RNAi process. The worms can even be fed bacteria that have been genetically engineered to express the double-stranded RNA, thus avoiding the difficulties of microinjection.
Transgenic worms can be produced by injecting DNA into the ovary, where the DNA becomes incorporated into the oocytes. Geneticists have created a special reporter gene that produces the jellyfish green fluorescent protein (GFP). When this reporter gene is injected into the ovary and becomes inserted into the worm genome, its expression produces GFP, which fluoresces green, allowing the expression of the gene to be easily observed (Figure 1).
830