11.1 Large Amounts of DNA are Packed into a Cell
The packaging of tremendous amounts of genetic information into the small space within a cell has been called the ultimate storage problem. Consider the chromosome of the bacterium E. coli, a single molecule of DNA with approximately 4.6 million base pairs. Stretched out straight, this DNA would be about 1000 times as long as the cell within which it resides (Figure 11.1). Human cells contain more than 6 billion base pairs of DNA, which would measure over 2 meters (over 6 feet) stretched end to end. Even DNA in the smallest human chromosome would stretch 14,000 times the length of the nucleus. Clearly, DNA molecules must be tightly packed to fit into such small spaces.
The structure of DNA can be considered at three hierarchical levels: the primary structure of DNA is its nucleotide sequence; the secondary structure is the double-stranded helix; and the tertiary structure refers to higher-order folding that allows DNA to be packed into the confined space of a cell.
CONCEPTS
Chromosomal DNA exists in the form of very long molecules that are tightly packed to fit into the small confines of a cell.
Supercoiling
One type of DNA tertiary structure is supercoiling, which takes place when the DNA helix is subjected to strain by being overwound or underwound. The lowest energy state for B-DNA (see Chapter 10) is when it has approximately 10 bp per turn of its helix. In this relaxed state, a stretch of 100 bp of DNA would assume about 10 complete turns (Figure 11.2a). If energy is used to add or remove any turns, strain is placed on the molecule, causing the helix to supercoil, or twist, on itself (Figure 11.2b and c). Molecules that are overrotated exhibit positive supercoiling (see Figure 11.2b). Underrotated molecules exhibit negative supercoiling (see Figure 11.2c). Supercoiling is a partial solution to the cell’s DNA packing problem because supercoiled DNA occupies less space than relaxed DNA.
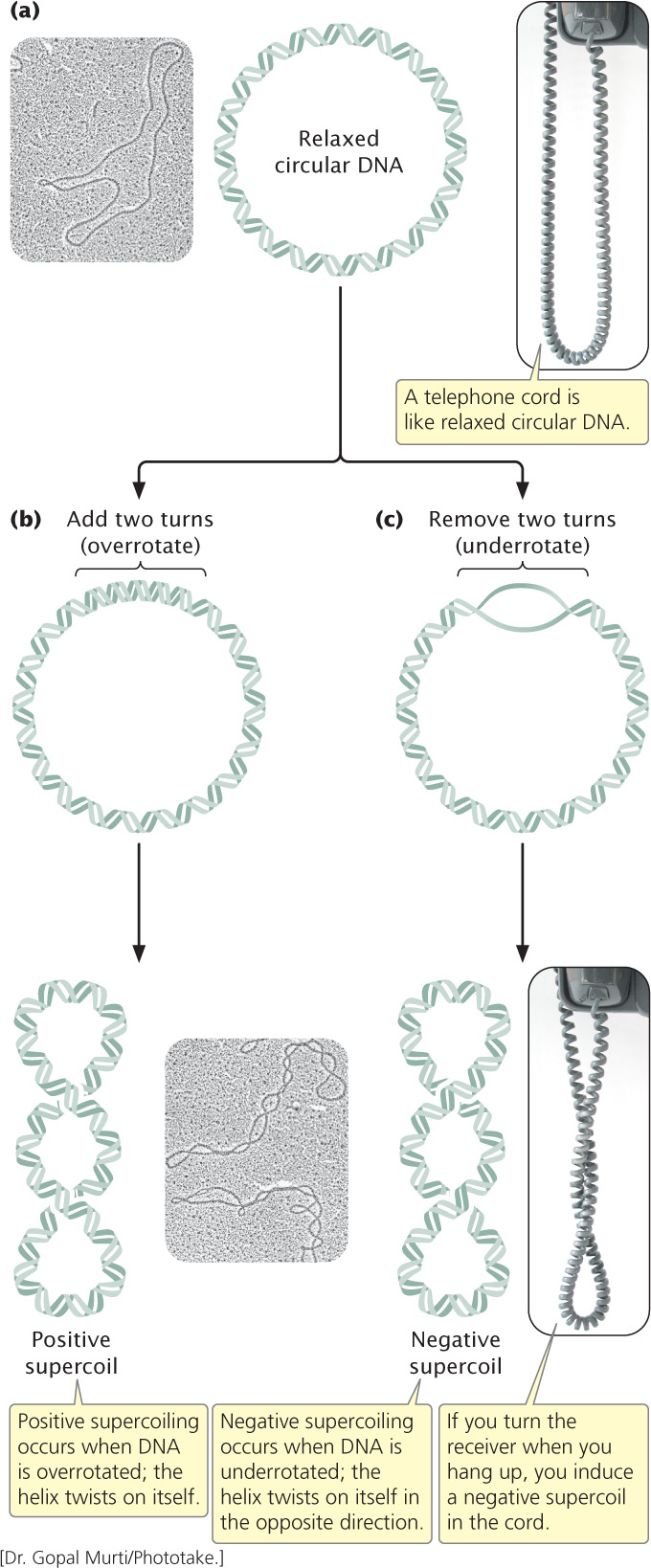
Supercoiling takes place when the strain of overrotating or underrotating cannot be compensated by the turning of the ends of the double helix, which is the case if the DNA is circular—that is, there are no free ends. If the chains can turn freely, their ends will simply turn as extra rotations are added or removed, and the molecule will spontaneously revert to the relaxed state. Both bacterial and eukaryotic DNA usually fold into loops stabilized by proteins (which prevent free rotation of the ends, see Figure 11.3), and supercoiling takes place within the loops.

Supercoiling relies on topoisomerases, enzymes that add or remove rotations from the DNA helix by temporarily breaking the nucleotide strands, rotating the ends around each other, and then rejoining the broken ends. Thus topoisomerases can both induce and relieve supercoiling, although not all topoisomerase enzymes do both.
301
Most DNA found in cells is negatively supercoiled, which has two advantages over nonsupercoiled DNA. First, negative supercoiling makes the separation of the two strands of DNA easier during replication and transcription. Negatively supercoiled DNA is underrotated, so separation of the two strands during replication and transcription is more rapid and requires less energy. Second, supercoiled DNA can be packed into a smaller space than can relaxed DNA.
CONCEPTS
Overrotation or underrotation of a DNA double helix places strain on the molecule, causing it to supercoil. Supercoiling is controlled by topoisomerase enzymes. Most cellular DNA is negatively supercoiled, which eases the separation of nucleotide strands during replication and transcription and allows DNA to be packed into small spaces.
 CONCEPT CHECK 1
CONCEPT CHECK 1
A DNA molecule 300 bp long has 20 complete rotations. This DNA molecule is
- positively supercoiled.
- negatively supercoiled.
- relaxed.
The Bacterial Chromosome
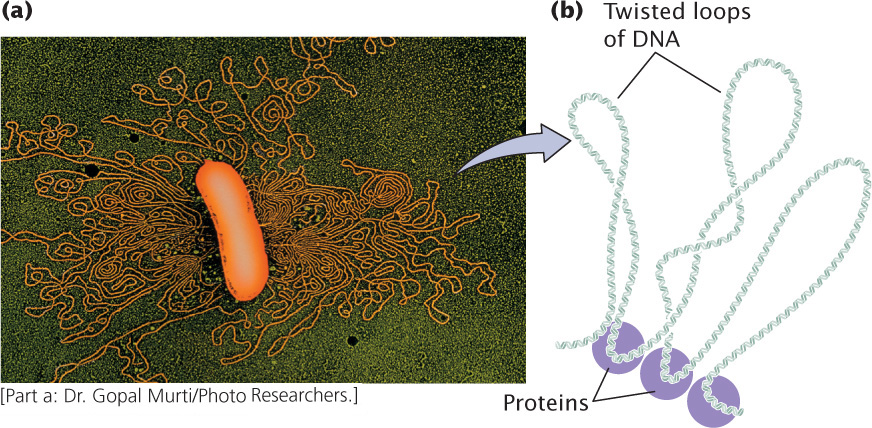
Most bacterial genomes consist of a single circular DNA molecule, although linear DNA molecules have been found in a few species. In circular bacterial chromosomes, the DNA does not exist in an open, relaxed circle; the 3 million to 4 million base pairs of DNA found in a typical bacterial genome would be much too large to fit into a bacterial cell (see Figure 11.1). Bacterial DNA is not attached to histone proteins as is eukaryotic DNA (discussed later in this chapter), but bacterial DNA is complexed to a number of proteins that help to compact it.
When a bacterial cell is viewed with the electron microscope, its DNA frequently appears as a distinct clump, the nucleoid, which is confined to a definite region of the cytoplasm. If a bacterial cell is broken open gently, its DNA spills out in a series of twisted loops (Figure 11.3a). The ends of the loops are most likely held in place by proteins (Figure 11.3b). Many bacteria contain additional DNA in the form of small circular molecules called plasmids, which replicate independently of the chromosome (see Chapter 9).
CONCEPTS
A typical bacterial chromosome consists of a large, circular molecule of DNA that is a series of twisted loops. Bacterial DNA appears as a distinct clump, the nucleoid, within the bacterial cell.
 CONCEPT CHECK 2
CONCEPT CHECK 2
How does bacterial DNA differ from eukaryotic DNA?
302
Eukaryotic Chromosomes
Individual eukaryotic chromosomes contain enormous amounts of DNA. Like the bacterial chromosome, each eukaryotic chromosome consists of a single, extremely long molecule of DNA. For this DNA to fit into the nucleus, tremendous packing and folding are required, the extent of which must change in the course of the cell cycle. The chromosomes are in an elongated, relatively uncondensed state during interphase of the cell cycle (see pp. 93–94 in Chapter 2), but the term relatively is important here. Although the DNA of interphase chromosomes is less tightly packed than the DNA of mitotic chromosomes, it is still highly condensed; it’s just less condensed. In the course of the cell cycle, the level of DNA packing changes: chromosomes progress from a highly packed state to a state of extreme condensation, which is necessary for chromosome movement in mitosis and meiosis. DNA packing also changes locally in replication and transcription, when the two nucleotide strands must unwind so that particular base sequences are exposed. Thus, the packing of eukaryotic DNA (its tertiary chromosomal structure) is not static but changes regularly in response to cellular processes.
Chromatin
Eukaryotic DNA in the cell is closely associated with proteins. This combination of DNA and protein is called chromatin. The two basic types of chromatin are euchromatin, which undergoes the normal process of condensation and decondensation in the cell cycle, and heterochromatin, which remains in a highly condensed state throughout the cell cycle, even during interphase. Euchromatin constitutes the majority of the chromosomal material and is where most transcription takes place. All chromosomes have permanent heterochromatin (called constitutive heterochromatin) at the centromeres and telomeres; the Y chromosome also consists largely of constitutive heterochromatin. Heterochromatin may also occur during certain developmental stages; this is referred to as facultative heterochromatin. For example, facultative heterochromatin occurs along one entire X chromosome in female mammals when this X becomes inactivated. In addition to remaining condensed throughout the cell cycle, heterochromatin is characterized by a general lack of transcription, the absence of crossing over, and replication late in the S phase. Differences between euchromatin and heterochromatin are summarized in Table 11.1.
| Characteristic | Euchromatin | Heterochromatin |
| Chromatin condensation | Less condensed | More condensed |
| Location | On chromosome arms | At centromeres, telomeres, and other specific places |
| Type of sequences | Unique sequences | Repeated sequences* |
| Presence of genes | Many genes | Few genes* |
| When replicated | Throughout S phase | Late S phase |
| Transcription | Often | Infrequent |
| Crossing over | Common | Uncommon |
| *Applies only to constitutive heterochromatin. | ||
The most abundant proteins in chromatin are the histones, which are small, positively charged proteins of five major types: H1, H2A, H2B, H3, and H4. All histones have a high percentage of arginine and lysine, positively charged amino acids that give the histones a net positive charge. The positive charges attract the negative charges on the phosphates of DNA; this attraction holds the DNA in contact with the histones. A heterogeneous assortment of nonhistone chromosomal proteins also are found in eukaryotic chromosomes. At times, variant histones, with somewhat different amino acid sequences, are incorporated into chromatin in place of one of the major histone proteins.  TRY PROBLEM 18
TRY PROBLEM 18
303
CONCEPTS
Chromatin, which consists of DNA complexed to proteins, is the material that makes up eukaryotic chromosomes. The most abundant of these proteins are the five types of positively charged histone proteins: H1, H2A, H2B, H3, and H4. Variant histones may at times be incorporated into chromatin in place of the normal histones.
 CONCEPT CHECK 3
CONCEPT CHECK 3
Neutralizing their positive charges would have which effect on the histone proteins?
- They would bind the DNA tighter.
- They would bind less tightly to the DNA.
- They would no longer be attracted to each other.
- They would cause supercoiling of the DNA.
The Nucleosome
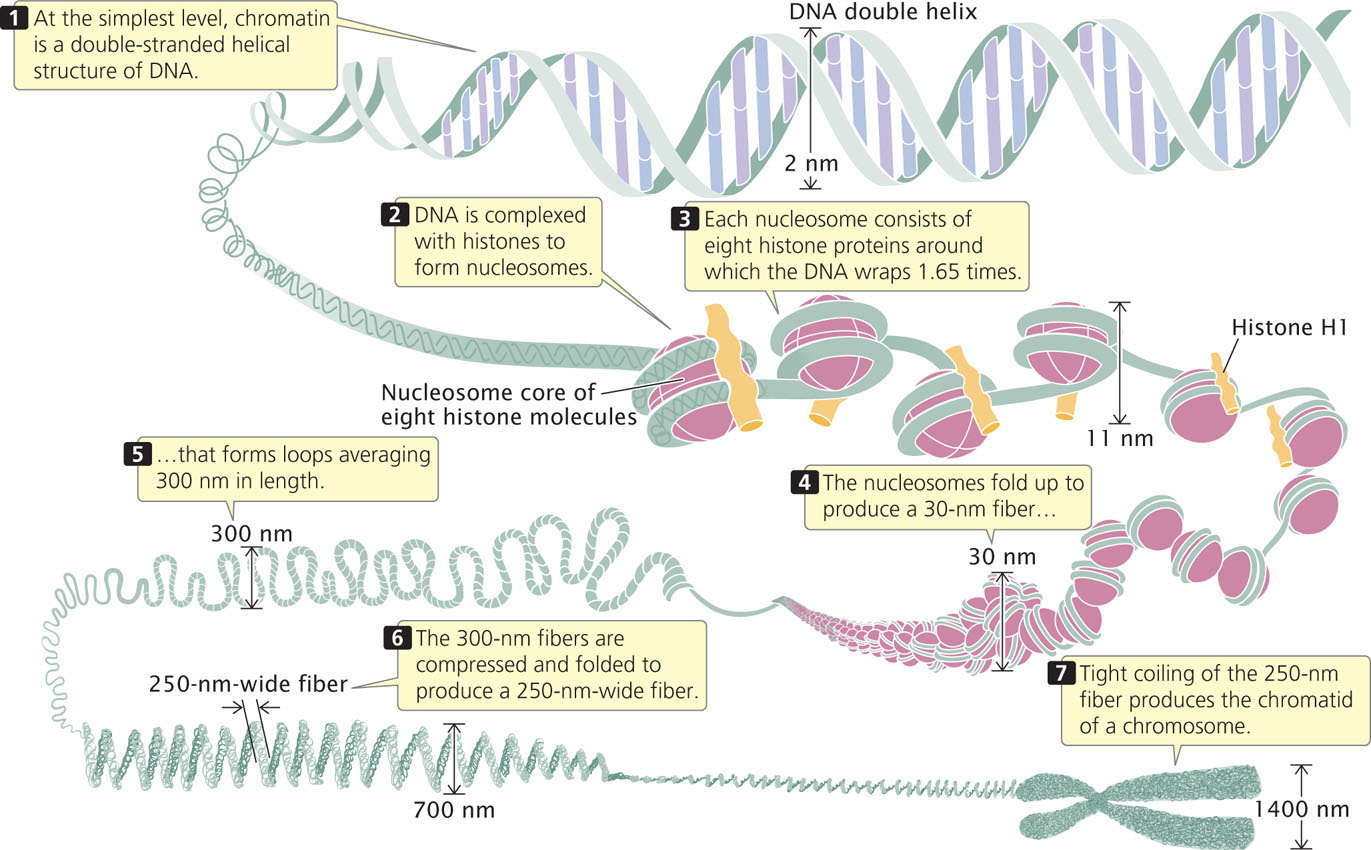
Chromatin has a highly complex structure with several levels of organization (Figure 11.4). The simplest level is the double-helical structure of DNA discussed in Chapter 10. At a more complex level, the DNA molecule is associated with proteins and is highly folded to produce a chromosome.
When chromatin is isolated from the nucleus of a cell and viewed with an electron microscope, it frequently looks like beads on a string (Figure 11.5a). If a small amount of nuclease is added to this structure, the enzyme cleaves the “string” between the “beads,” leaving individual beads attached to about 200 bp of DNA (Figure 11.5b). If more nuclease is added, the enzyme chews up all of the DNA between the beads and leaves a core of proteins attached to a fragment of DNA (Figure 11.5c). Such experiments demonstrated that chromatin is not a random association of proteins and DNA but has a fundamental repeating structure.
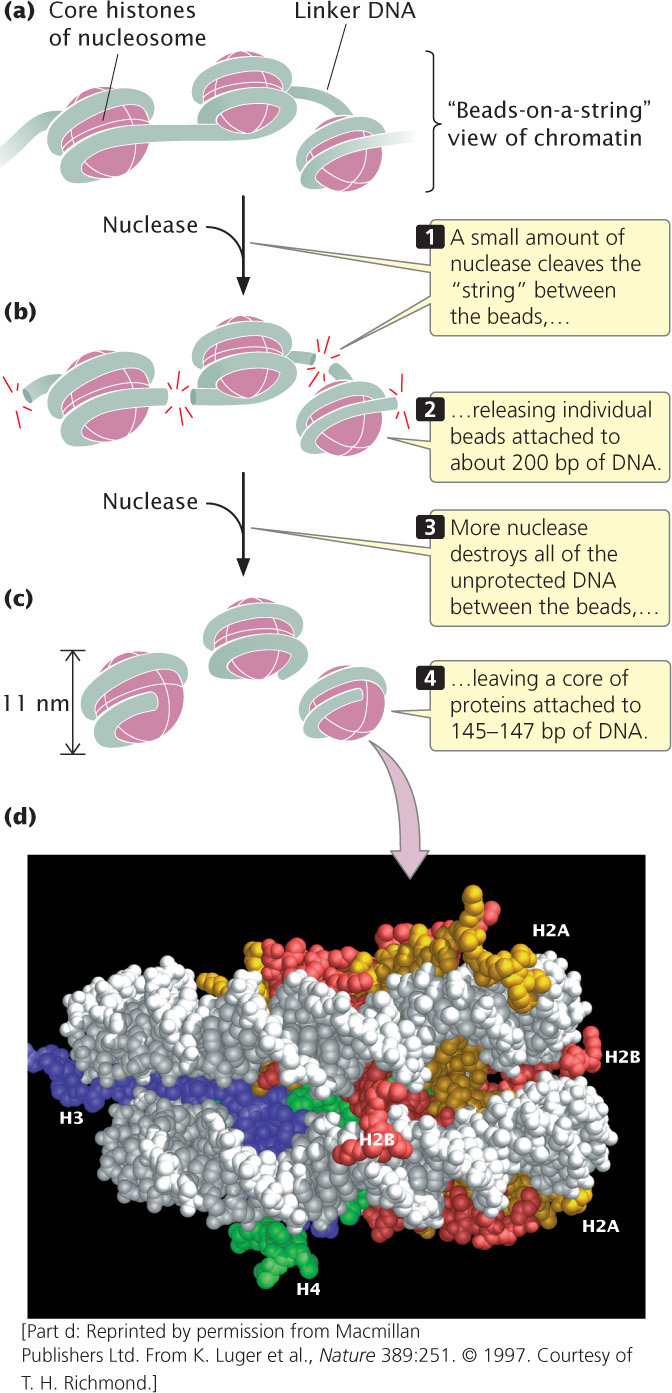
The repeating core of protein and DNA produced by digestion with nuclease enzymes is the simplest level of chromatin structure, the nucleosome (see Figure 11.4). The nucleosome is a core particle consisting of DNA wrapped about two times around an octamer of eight histone proteins (two copies each of H2A, H2B, H3, and H4), much like thread wound around a spool (Figure 11.5d). The DNA in direct contact with the histone octamer is between 145 and 147 bp in length.
Each of the histone proteins that make up the nucleosome core particle has a flexible “tail,” containing from 11 to 37 amino acids, which extends out from the nucleosome. Positively charged amino acids in the tails of the histones interact with the negative charges of the phosphates on the DNA, keeping the DNA and histones tightly associated. The tails of one nucleosome may also interact with neighboring nucleosomes, which facilitates compaction of the nucleosomes themselves. Chemical modifications of the histone tails bring about changes in chromatin structure (discussed in the next section) that are necessary for gene expression.
304
The fifth type of histone, H1, is not a part of the nucleosome core particle but plays an important role in nucleosome structure. H1 binds to 20 to 22 bp of DNA where the DNA joins and leaves the octamer (see Figure 11.4) and helps to lock the DNA into place, acting as a clamp around the nucleosome octamer.
Each nucleosome encompasses about 167 bp of DNA. Nucleosomes are located at regular intervals along the DNA molecule and are separated from one another by linker DNA, which varies in size among cell types; in most cells, linker DNA comprises from about 30 to 40 bp. Nonhistone chromosomal proteins may be associated with this linker DNA, and a few also appear to bind directly to the core particle.  TRY PROBLEMS 19 AND 22
TRY PROBLEMS 19 AND 22
Higher-Order Chromatin Structure
When chromatin is in a condensed form, adjacent nucleosomes are not separated by space equal to the length of the linker DNA; rather, nucleosomes fold on themselves to form a dense, tightly packed structure (see Figure 11.4) that makes up a fiber with a diameter of about 30 nm. The precise molecular structure of the 30 nm fiber remains uncertain.
The next-higher level of chromatin structure is a series of loops of 30-nm fibers (Figure 11.4), each anchored at its base by proteins. On average, each loop encompasses some 20,000 to 100,000 bp of DNA and is about 300 nm in length, but the individual loops vary considerably. The 300-nm loops are packed and folded to produce a 250-nm-wide fiber. Tight helical coiling of the 250-nm fiber, in turn, produces the structure that appears in metaphase—individual chromatids approximately 700 nm in width. You can view the different levels of chromatin structure in Animation 11.1.
CONCEPTS
The nucleosome consists of a core particle of eight histone proteins and DNA that wraps around the core. A single H1 histone associates with each core particle. Nucleosomes are separated by linker DNA. Nucleosomes fold to form a 30-nm chromatin fiber, which appears as a series of loops that pack to create a 250-nm-wide fiber. Helical coiling of the 250-nm fiber produces a chromatid.
 CONCEPT CHECK 4
CONCEPT CHECK 4
How many copies of the H2B histone would be found in chromatin containing 50 nucleosomes?
- 5
- 10
- 50
- 100
Changes in Chromatin Structure
Although eukaryotic DNA must be tightly packed to fit into the cell nucleus, it must also periodically unwind to undergo transcription and replication.
Polytene Chromosomes
Giant chromosomes called polytene chromosomes are found in certain tissues of Drosophila and some other organisms (Figure 11.6). Polytene chromosomes have provided researchers with evidence of the changing nature of chromatin structure. These large, unusual chromosomes arise when repeated rounds of DNA replication take place without accompanying cell divisions, producing thousands of copies of DNA that lie side by side. When polytene chromosomes are stained with dyes, numerous bands are revealed. Under certain conditions, the bands may exhibit chromosomal puffs—localized swellings of the chromosome. Each puff is a region of the chromatin that has a relaxed structure and, consequently, a more open state. Research indicates that chromosomal puffs are regions of active transcription. This correlation between the occurrence of transcription and the relaxation of chromatin at a puff site indicates that chromatin structure undergoes dynamic change associated with gene activity.  TRY PROBLEM 20
TRY PROBLEM 20
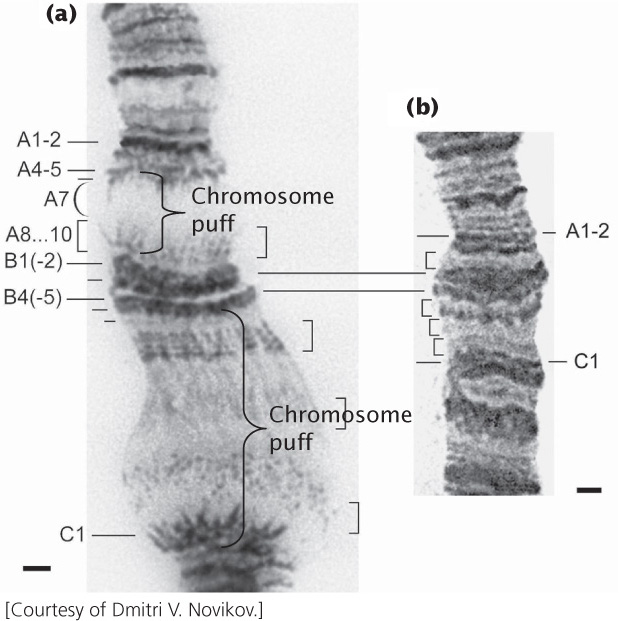
305
DNase I Sensitivity
A second piece of evidence indicating that chromatin structure changes with gene activity is sensitivity to DNase I, an enzyme that digests DNA. The ability of this enzyme to digest DNA depends on chromatin structure: when DNA is tightly bound to histone proteins, it is less sensitive to DNase I, whereas unbound DNA is more sensitive to DNase I. The results of experiments that examine the effect of DNase I on specific genes show that DNase sensitivity is correlated with gene activity. Studies of chicken globin genes give evidence for this correlation. Globin genes encode hemoglobin in the erythroblasts (precursors of red blood cells) of chickens (Figure 11.7). These types of experiments demonstrate that transcriptionally active genes are sensitive to DNase I, indicating that the chromatin structure is more exposed during transcription.
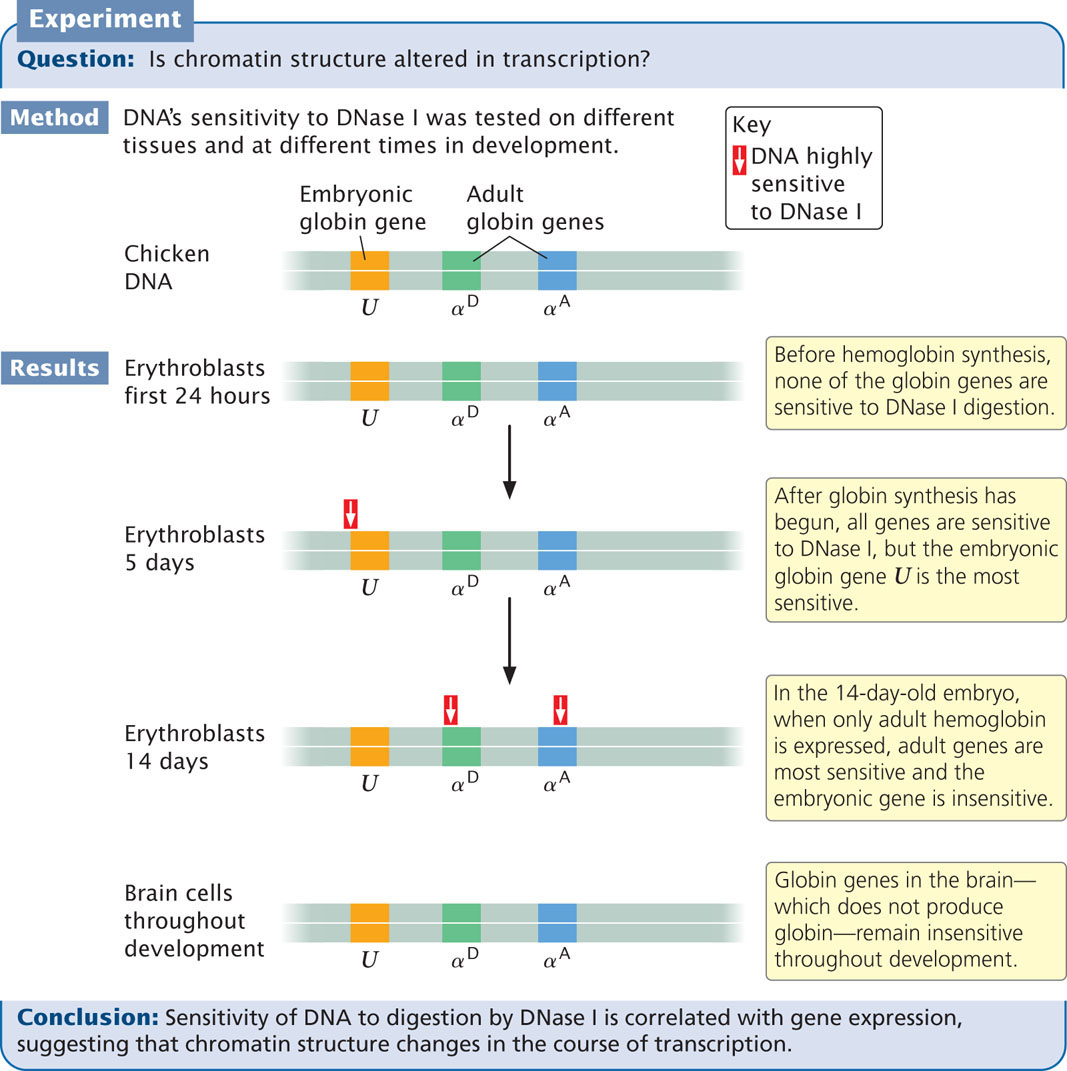
306
What is the nature of the change in chromatin structure that produces chromosome puffs and DNase I sensitivity? In both cases, the chromatin relaxes; presumably, the histones loosen their grip on the DNA. One process that alters chromatin structure is acetylation. Enzymes called acetyltransferases attach acetyl groups to lysine amino acids on the histone tails. This modification reduces the positive charges that normally exist on lysine and destabilizes the nucleosome structure, and so the histones hold the DNA less tightly. Other chemical modifications of the histone proteins, such as methylation and phosphorylation, also alter chromatin structure, as do special chromatin-remodeling proteins that bind to the DNA.
Epigenetic Changes Associated With Chromatin Modifications
We have now seen how chromatin structure can be altered by chemical modification of the histone proteins. A number of other changes can also affect chromatin structure, including the methylation of DNA (see Chapter 10), the use of variant histone proteins in the nucleosome, and the binding of proteins to DNA and chromatin. Although these changes do not alter the DNA sequence, they often have major effects on the expression of genes, which will be discussed in more detail in Chapter 17.
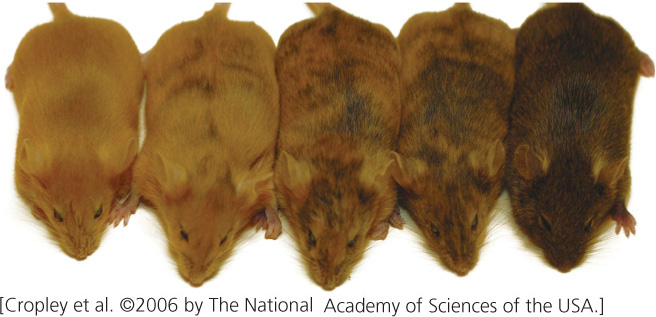
Some changes to chromatin structure are retained through cell division, and so they are passed on to future generations of cells and even occasionally to future generations of organisms. Stable alterations of chromatin structure that may be passed on to cells or individual organisms are frequently referred to as epigenetic changes or simply as epigenetics (see Chapter 5). For example, the agouti locus helps determine coat color in mice: parents that have identical DNA sequences but have different degrees of methylation on their DNA may give rise to offspring with different coat colors (Figure 11.8). Such epigenetic changes have been observed in a number of organisms and are responsible for a variety of phenotypic effects. Unlike mutations, epigenetic changes do not alter the DNA sequence, are capable of being reversed, and are often influenced by environmental factors.
CONCEPTS
Epigenetic changes are alterations of chromatin or DNA structure that do not include changes in the base sequence but are stable and passed on to cells or organisms. Some epigenetic changes result from alterations of histone proteins.