14.5 Small RNA Molecules Participate in a Variety of Functions
Much evidence suggests that the first genetic material was RNA and early life was dominated by RNA molecules (see Chapter 13). This time period, when RNA dominated life’s essential processes, has been termed the “early RNA world.” The common perception is that this RNA world died out billions of years ago, when many of RNA’s functions were replaced by more-stable DNA molecules and more-efficient protein catalysts. However, within the past 10 years, numerous small RNA molecules (most of them 20–30 nucleotides long) have been discovered that greatly influence many basic biological processes, including the formation of chromatin structure, transcription, and translation. These small RNA molecules play important roles in gene expression, development, cancer, and defense against foreign DNA. They are also being harnessed by researchers to study gene function and treat genetic diseases. The discovery of small RNA molecules has greatly influenced our understanding of how genes are regulated and the importance of DNA sequences that do not encode proteins. These new findings demonstrate that we still live very much in an RNA world.
In this section, we will examine RNA interference (which led to the discovery of small RNAs), different types of small RNAs, and how miRNAs are processed. In Chapter 17, we will look further at the role of small RNAs in controlling gene expression; in Chapter 19, we will see how small RNAs are being used as important tools in biotechnology.
RNA Interference
In 1998, Andrew Fire, Craig Mello, and their colleagues observed a strange phenomenon. They were inhibiting the expression of genes in the nematode Caenorhabditis elegans by inserting single-stranded RNA molecules that were complementary to a gene’s DNA sequence. Called antisense RNA, such molecules are known to inhibit gene expression by binding to the mRNA sequences and inhibiting translation. Fire, Mello, and colleagues found that even more potent gene silencing was triggered when double-stranded RNA was injected into the animals. This finding was puzzling, because no mechanism by which double-stranded RNA could inhibit translation was known. Several other, previously described types of gene silencing also were found to be triggered by double-stranded RNA. These initial studies led to the discovery of small RNA molecules that are important in gene silencing.
Subsequent research revealed an astonishing array of small RNA molecules with important cellular functions in eukaryotes, which now include at least three major classes: small interfering RNAs (siRNAs), microRNAs (miRNAs), and Piwi-interacting RNAs (piRNAs), depending on their origin and mode of function. These small RNAs are found in many eukaryotes and are responsible for a variety of different functions, including the regulation of gene expression, defense against viruses, suppression of transposons, and modification of chromatin structure. An analogous group of small RNAs with silencing functions—called CRISPR RNAs (crRNAs)—have been detected in prokaryotes. For their discovery of RNA interference, Fire and Mello were awarded the Nobel Prize in physiology or medicine in 2006.
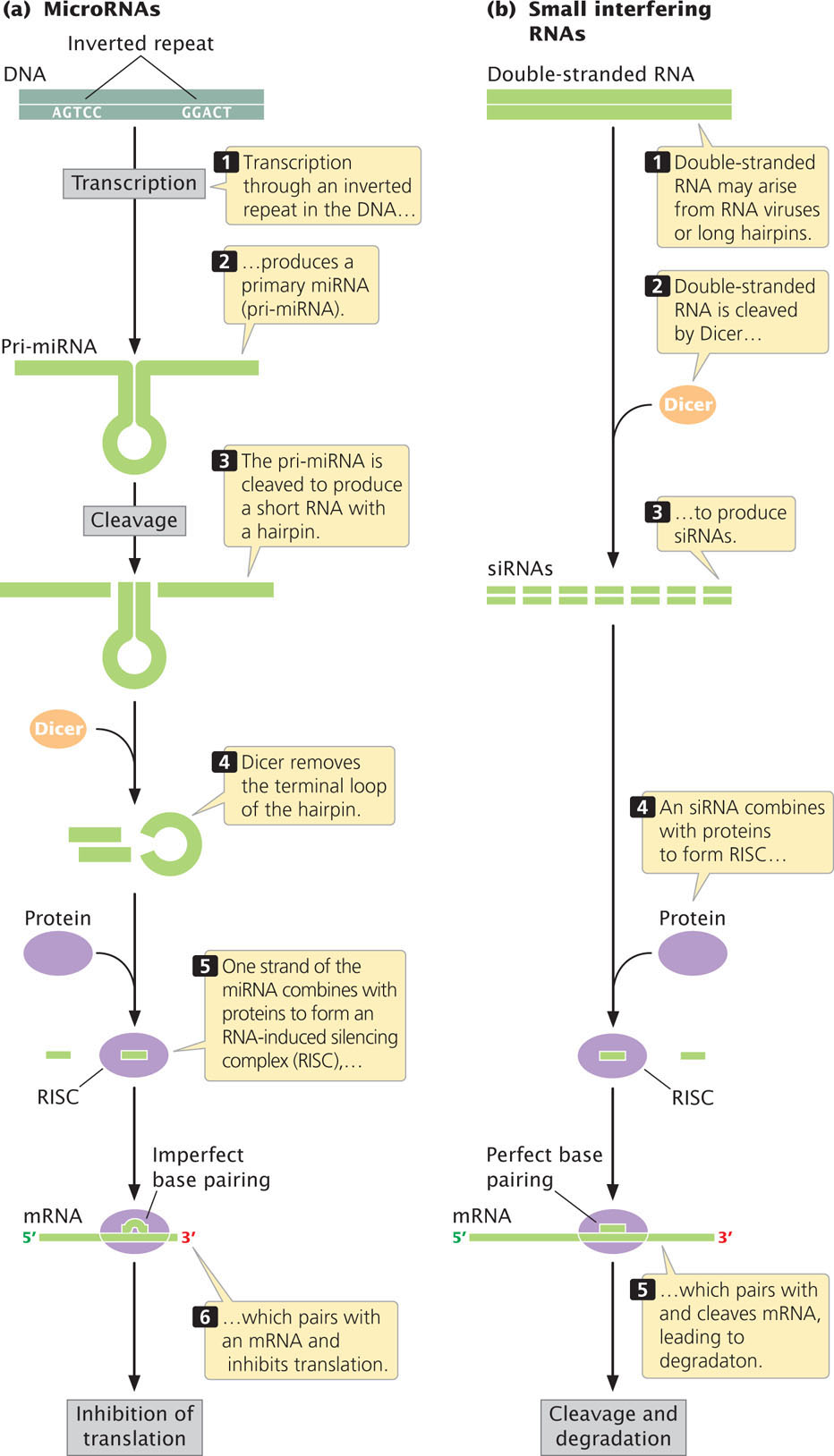
RNA interference (RNAi) is a powerful and precise mechanism used by eukaryotic cells to limit the invasion of foreign genes (from viruses and transposons) and to censor the expression of their own genes. RNA interference is triggered by double-stranded RNA molecules, which may arise in several ways (Figure 14.22): by the transcription of inverted repeats into an RNA molecule that then base pairs with itself to form double-stranded RNA; by the simultaneous transcription of two different RNA molecules that are complementary to one another and that pair, forming double-stranded RNA; or by infection by viruses that make double-stranded RNA. These double-stranded RNA molecules are chopped up by an enzyme appropriately called Dicer, resulting in tiny RNA molecules that are unwound to produce siRNAs and miRNAs (see Figure 14.22).
403
Some geneticists speculate that RNA interference evolved as a defense mechanism against RNA viruses and transposable elements that move through RNA intermediates (see Chapter 18); indeed, some have called RNAi the immune system of the genome. However, RNA interference is also responsible for regulating a number of key genetic and developmental processes, including changes in chromatin structure, translation, cell fate and proliferation, and cell death. Geneticists also use the RNAi machinery as an effective tool for blocking the expression of specific genes (see Chapter 19).
Small Interfering and Micro RNAs
Two abundant classes of RNA molecules that function in RNA interference in eukaryotes are small interfering RNAs and microRNAs. Although these two types of RNA differ in how they originate (Table 14.5; see Figure 14.22), they have a number of features in common and their functions overlap considerably. Both are about 22 nucleotides long. Small interfering RNAs arise from the cleavage of mRNAs, RNA transposons, and RNA viruses. Some miRNAs are cleaved from RNA molecules transcribed from sequences that encode miRNA only, but others are encoded in the introns and exons of mRNAs. Each miRNA is cleaved from a single-stranded RNA precursor that forms small hairpins, whereas multiple siRNAs are produced from the cleavage of an RNA duplex consisting of two different RNA molecules.
| Feature | siRNA | miRNA |
|---|---|---|
| Origin | mRNA, transposon, or virus | RNA transcribed from distinct gene |
| Cleavage of | RNA duplex or single-stranded RNA that forms long hairpins | Single-stranded RNA that forms short hairpins of double-stranded RNA |
| Size | 21–25 nucleotides | 21–25 nucleotides |
| Action | Degradation of mRNA, inhibition of transcription, chromatin modification | Degradation of mRNA, inhibition of translation, chromatin modification |
| Target | Genes from which they were transcribed | Genes other than those from which they were transcribed |
Usually, siRNAs have exact complementarity with their target mRNA or DNA sequences, whereas miRNAs often have limited complementarity with their target mRNAs. Small interfering RNAs suppress gene expression by degrading mRNA or inhibiting transcription, while miRNAs often suppress gene expression by inhibiting translation. Finally, miRNAs usually silence genes that are distinct from those from which the miRNAs were transcribed, whereas siRNAs typically silence the genes from which the siRNAs were transcribed. Note, however, that these differences between siR-NAs and miRNAs are not hard and fast, and scientists are increasingly finding small RNAs that exhibit characteristics of both. For example, some miRNAs (such as those in plants) have exact complementarity with mRNA sequences and cleave these sequences, characteristics that are usually associated with siRNAs.
Both siRNA and miRNA molecules combine with proteins to form an RNA-induced silencing complex (RISC; see Figure 14.22). Key to the functioning of RISCs is a protein called Argonaute. The RISC pairs with an mRNA molecule that possesses a sequence complementary to its siRNA or miRNA component and either cleaves the mRNA, leading to degradation of the mRNA, or represses translation of the mRNA. Some siRNAs also serve as guides for the methylation of complementary sequences in DNA and others alter chromatin structure, both of which affect transcription. To see how small interfering RNAs and microRNAs affect gene expression, see  Animation 14.3.
Animation 14.3.
MicroRNAs have been found in all eukaryotic organisms examined to date, as well as viruses: they control the expression of genes taking part in many biological processes, including growth, development, and metabolism. Humans have more than 450 distinct miRNAs; scientists estimate that more than one-third of all human genes are regulated by miRNAs. Most miRNA genes are found in regions of non-coding DNA or within the introns of other genes.
Processing and Function of miRNAs and siRNAs
The genes that encode miRNAs are transcribed into longer precursors, called primary miRNA (pri-miRNA), that range from several hundred to several thousand nucleotides in length (Figure 14.22a). The pri-miRNA is then cleaved into one or more smaller RNA molecules with a hairpin. Dicer binds to this hairpin structure and removes the terminal loop. One of the miRNA strands is incorporated into the RISC; the other strand is released and degraded.
404
The RISC attaches to a complementary sequence on the mRNA, usually in the 3′ untranslated region of the mRNA. The region of close complementarity, called the seed region, is quite short, usually only about seven nucleotides long. Because the seed sequence is so short, each miRNA can potentially pair with sequences on hundreds of different mRNAs. Furthermore, a single mRNA molecule may possess multiple miRNA-binding sites. The inhibition of translation may require binding by several RISC complexes to the same mRNA molecule.  Animation 14.2 illustrates the process by which microRNAs are produced.
Animation 14.2 illustrates the process by which microRNAs are produced.
Small interfering RNAs are processed in a similar way (Figure 14.22b). Double-stranded RNA from viruses or long hairpins is cleaved by Dicer to produce siRNAs, which combine with proteins to form a RISC. The RISC then pairs with sequences on target mRNA and cleaves the mRNA, after which the mRNA is degraded.
CONCEPTS
Small interfering RNAs and microRNAs are tiny RNAs produced when larger, double-stranded RNA molecules are cleaved by the enzyme Dicer. Small interfering RNAs and microRNAs participate in a variety of processes, including mRNA degradation, the inhibition of translation, the methylation of DNA, and chromatin remodeling.
 CONCEPT CHECK 9
CONCEPT CHECK 9
How do siRNAs and miRNAs target specific mRNAs for degradation or for the repression of translation?
Piwi-Interacting RNAs
Piwi-interacting RNAs (piRNAs) were discovered in 2001. They are somewhat longer than siRNAs and miRNAs, consisting of 24 to 30 nucleotides, and are derived from long, single-stranded RNA transcripts, in contrast with siRNAs and miRNAs, which are processed from double-stranded RNA. Also unlike siRNAs and miRNAs, Dicer is not involved in the production of piRNAs.
Piwi-interacting RNAs combine with Piwi proteins, which are related to Argonaute, and suppress the expression and movement of transposons in the germ cells of animals. Although the mechanism of transposon silencing by piRNAs is not fully understood, we know that it includes the degradation of mRNA transcribed from transposons, changes in chromatin structure that inhibit the transcription of transposons, and inhibition of the translation of proteins encoded by transposons. What, if any, function piRNAs have outside of germ cells is not known.
CRISPR RNA
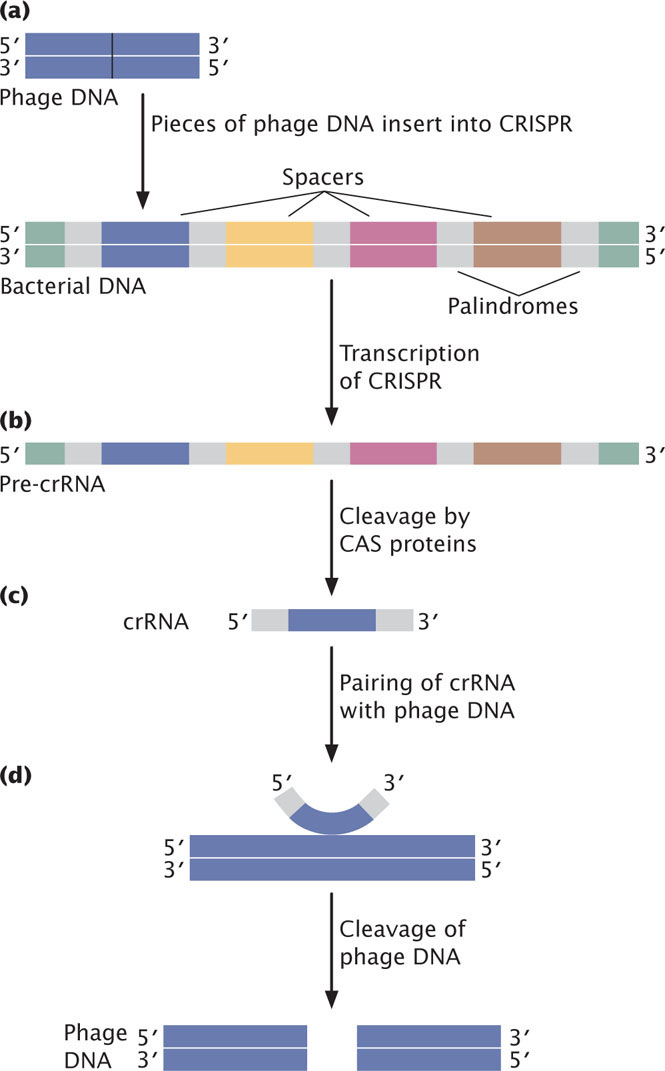
After the discovery of small RNAs in eukaryotes, similar small RNAs called CRISPR RNAs (crRNAs) were discovered in prokaryotes: crRNAs are encoded by DNA sequences found in bacterial and archaeal genomes termed Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR). Palindromic sequences are sequences that read the same forwards and backwards on two complementary DNA strands. CRISPR consists of a series of such palindromic sequences, separated by unique sequences that are homologous to DNA from bacteriophage or plasmid genomes. For example, in the bacterium Pseudomonas auruginosa, CRISPR palindromic repeats are 28 bp in length, separated by 32 bp spacers.
CRISPR RNAs play a role in defense against the invasion of specific foreign DNAs, such as DNA originating from bacteriophage and plasmids (see Chapter 9). Because they target specific DNA molecules, the CRISPR system has been compared to the immune system of vertebrates.
Through a mechanism that is still incompletely understood, when a bacteriophage or plasmid invades a prokaryotic cell, small portions of the invader genome are inserted as spacers between the palindromic repeats in CRISPR (Figure 14.23a). The spacer DNA then serves as a memory of this invader’s DNA. The CRISPR region is transcribed into a single long CRISPR precursor RNA (Figure 14.23b), which is then cleaved by CRISPR-associated proteins (Cas proteins) into crRNAs, each of which consists of a spacer sequence (homologous to the invader’s DNA) flanked by a part of the palindromic sequence (Figure 14.23c). When additional foreign DNA from the same source enters the cell, crRNAs pair with it and help bring about cleavage of the invader DNA (Figure 14.23d). In this way, crRNAs serve as an adaptive, RNA defense system against foreign invaders.
405
CONCEPTS
Piwi-interacting RNAs are found in the germ cells of animals and inhibit transposons. CRISPR RNAs are found in prokaryotes, where they function in defense against foreign DNA.