15.1 Many Genes Encode Proteins
The first person to suggest the existence of a relation between genotype and proteins was English physician Archibald Garrod. In 1908, Garrod correctly proposed that genes encode enzymes, but, unfortunately, his theory made little impression on his contemporaries. Not until the 1940s, when George Beadle and Edward Tatum examined the genetic basis of biochemical pathways in Neurospora, did the relation between genes and proteins become widely accepted. Beadle and Tatum’s work helped define the relation between genotype and phenotype by leading to the one gene, one enzyme hypothesis, the idea that each gene encodes a separate enzyme.
The One Gene, One Enzyme Hypothesis
Beadle and Tatum used the bread mold Neurospora to study the biochemical results of mutations. Neurospora is easy to cultivate in the laboratory, and the main vegetative part of the fungus is haploid, which allows the effects of recessive mutations to be easily observed (Figure 15.1).
Wild-type Neurospora grows on minimal medium, which contains only inorganic salts, nitrogen, a carbon source such as sucrose, and the vitamin biotin. The fungus can synthesize all the biological molecules that it needs from these basic compounds. However, mutations may arise that disrupt fungal growth by destroying the fungus’s ability to synthesize one or more essential biological molecules. These nutritionally deficient mutants, termed auxotrophs (see Chapter 9), will not grow on minimal medium, but they can grow on medium that contains the substance that they are no longer able to synthesize.
Beadle and Tatum first irradiated spores of Neurospora to induce mutations (Figure 15.2). Then they placed individual spores into different culture tubes containing complete medium (medium having all the biological substances needed for growth). These spores grew into fungi and produced spores by mitosis. Next, they transferred spores from each culture to tubes containing minimal medium. Fungi containing auxotrophic mutations grew on complete medium but would not grow on minimal medium, which allowed Beadle and Tatum to identify cultures that contained mutations.
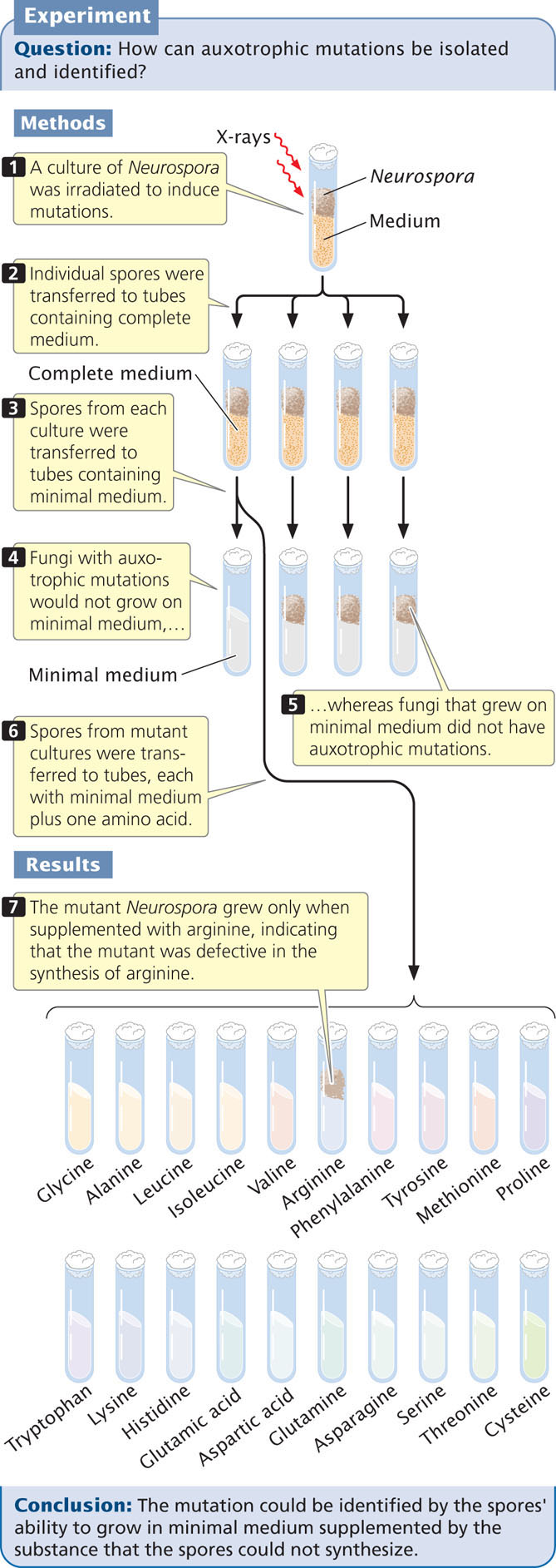
After they had determined that a particular culture had an auxotrophic mutation, Beadle and Tatum set out to determine the specific effect of the mutation. They transferred spores of each mutant strain from complete medium to a series of tubes (see Figure 15.2), each of which possessed minimal medium plus one of a variety of essential biological molecules, such as an amino acid. If the spores in a tube grew, Beadle and Tatum were able to identify the added substance as the biological molecule whose synthesis had been affected by the mutation. For example, an auxotrophic mutant that would grow only on minimal medium to which arginine had been added must have possessed a mutation that disrupts the synthesis of arginine.
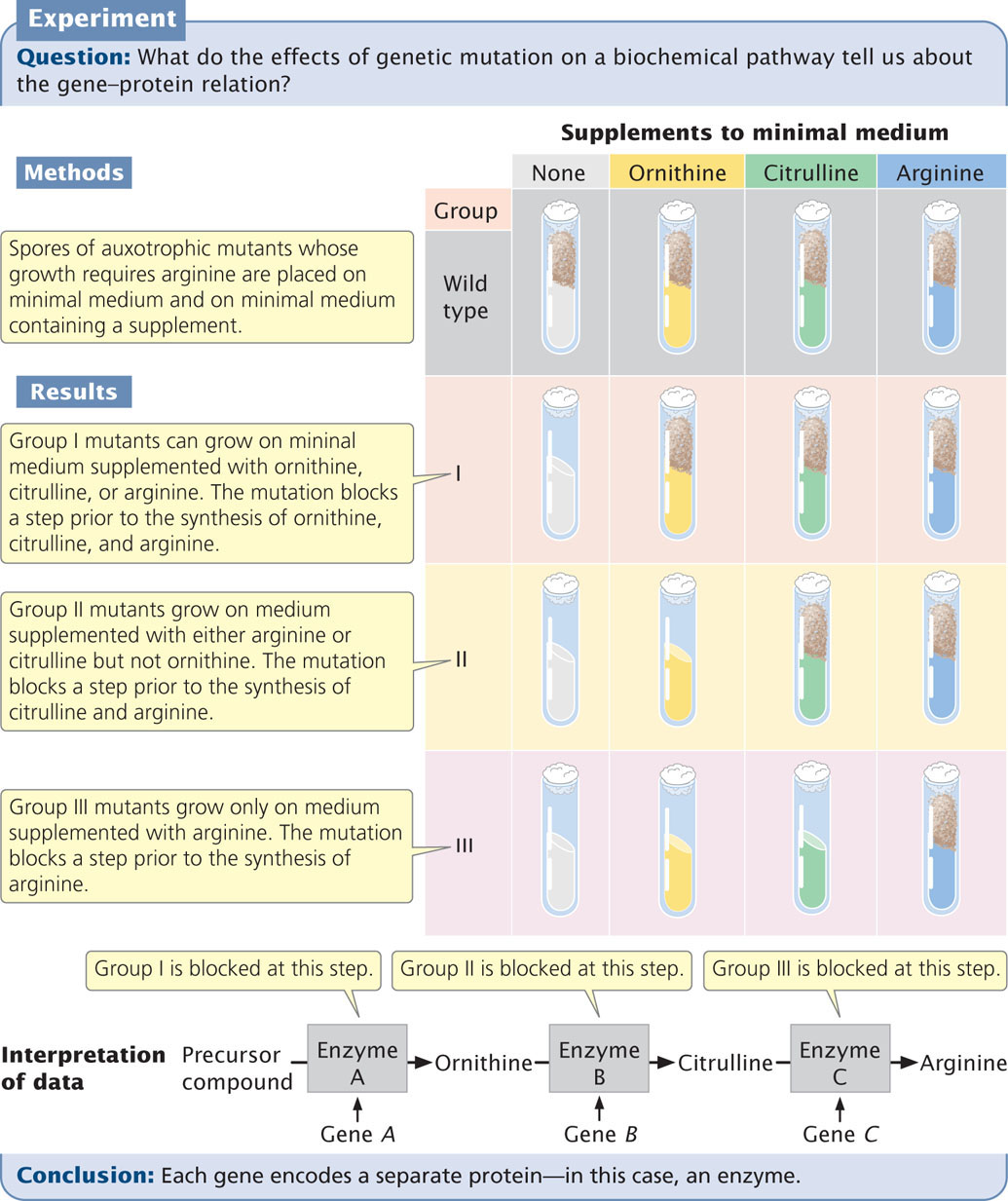
Adrian Srb and Norman H. Horowitz patiently applied this procedure to genetically dissect the multistep biochemical pathway of arginine synthesis (Figure 15.3). They first isolated a series of auxotrophic mutants whose growth required arginine. Then they tested these mutants for their ability to grow on minimal medium supplemented with three compounds: ornithine, citrulline, and arginine. From the results, they were able to place the mutants into three groups on the basis of which of the substances allowed growth (Table 15.1).
413
Based on these results, Srb and Horowitz proposed that the biochemical pathway leading to the amino acid arginine has at least three steps:
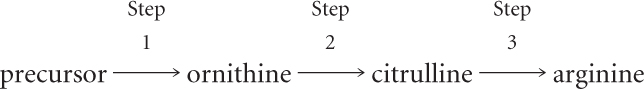
They concluded that the mutations in group I affect step 1 of this pathway, mutations in group II affect step 2, and mutations in group III affect step 3. But how did they know that the order of the compounds in the biochemical pathway was correct?
Notice that if step 1 is blocked by a mutation, then the addition of either ornithine or citrulline allows growth, because these compounds can still be converted into arginine (see Figure 15.3). Similarly, if step 2 is blocked, the addition of citrulline allows growth, but the addition of ornithine has no effect. If step 3 is blocked, the spores will grow only if arginine is added to the medium. The underlying principle is that an auxotrophic mutant cannot synthesize any compound that comes after the step blocked by a mutation.
Using this reasoning with the information in Table 15.1, we can see that the addition of arginine to the medium allows all three groups of mutants to grow. Therefore, biochemical steps affected by all the mutants precede the step that results in arginine. The addition of citrulline allows group I and group II mutants to grow but not group III mutants; therefore, group III mutations must affect a biochemical step that takes place after the production of citrulline but before the production of arginine.
| Mutant Strain Number | Ornithine | Citrulline | Arginine |
|---|---|---|---|
| Group I | + | + | + |
| Group II | − | + | + |
| Group III | − | − | + |
| Note: A plus sign (+) indicates growth; a minus sign (−) indicates no growth. | |||
414

The addition of ornithine allows the growth of group I mutants but not group II or group III mutants; thus, mutations in groups II and III affect steps that come after the production of ornithine. We’ve already established that group II mutations affect a step before the production of citrulline; so group II mutations must block the conversion of ornithine into citrulline.
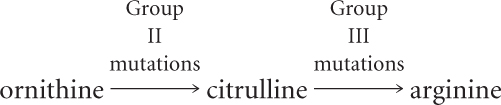
Because group I mutations affect some step before the production of ornithine, we can conclude that they must affect the conversion of some precursor into ornithine. We can now outline the biochemical pathway yielding ornithine, citrulline, and arginine.

Importantly, this procedure does not necessarily detect all steps in a pathway; rather, it only detects the steps that produce the compounds tested.
Using mutations and this type of reasoning, Beadle, Tatum, and others were able to identify genes that control several biosynthetic pathways in Neurospora. They established that each step in a pathway is controlled by a different enzyme, as shown in Figure 15.3 for the arginine pathway. They also conducted genetic crosses and mapping experiments (see Chapter 7) and were able to demonstrate that mutations affecting any one step in a pathway always occurred at the same chromosomal location. Beadle and Tatum reasoned that mutations affecting a particular biochemical step occurred at a single locus that encoded a particular enzyme. This idea became known as the one gene, one enzyme hypothesis: genes function by encoding enzymes, and each gene encodes a separate enzyme. Although the genes Beadle and Tatum examined encoded enzymes, many genes encode proteins that are not enzymes, so more generally their idea was that each gene encodes a protein. When research findings showed that some proteins are composed of more than one polypeptide chain and that different polypeptide chains are encoded by separate genes, this model was modified to become the one gene, one polypeptide hypothesis.  TRY PROBLEM 16
TRY PROBLEM 16
415
CONCEPTS
Beadle and Tatum’s studies of biochemical pathways in the fungus Neurospora helped define the relation between genotype and phenotype by establishing the one gene, one enzyme hypothesis, the idea that each gene encodes a separate enzyme. This was later modified to the one gene, one polypeptide hypothesis.
 CONCEPT CHECK 1
CONCEPT CHECK 1
Auxotrophic mutation 103 grows on minimal medium supplemented with A, B, or C; mutation 106 grows on medium supplemented with A and C but not B; and mutation 102 grows only on medium supplemented with C. What is the order of A, B, and C in a biochemical pathway?
The Structure and Function of Proteins
Proteins are central to all living processes (Figure 15.4). Many proteins are enzymes, the biological catalysts that drive the chemical reactions of the cell; others are structural components, providing scaffolding and support for membranes, filaments, bone, and hair. Some proteins help transport substances; others have a regulatory, communication, or defense function.

Amino Acids
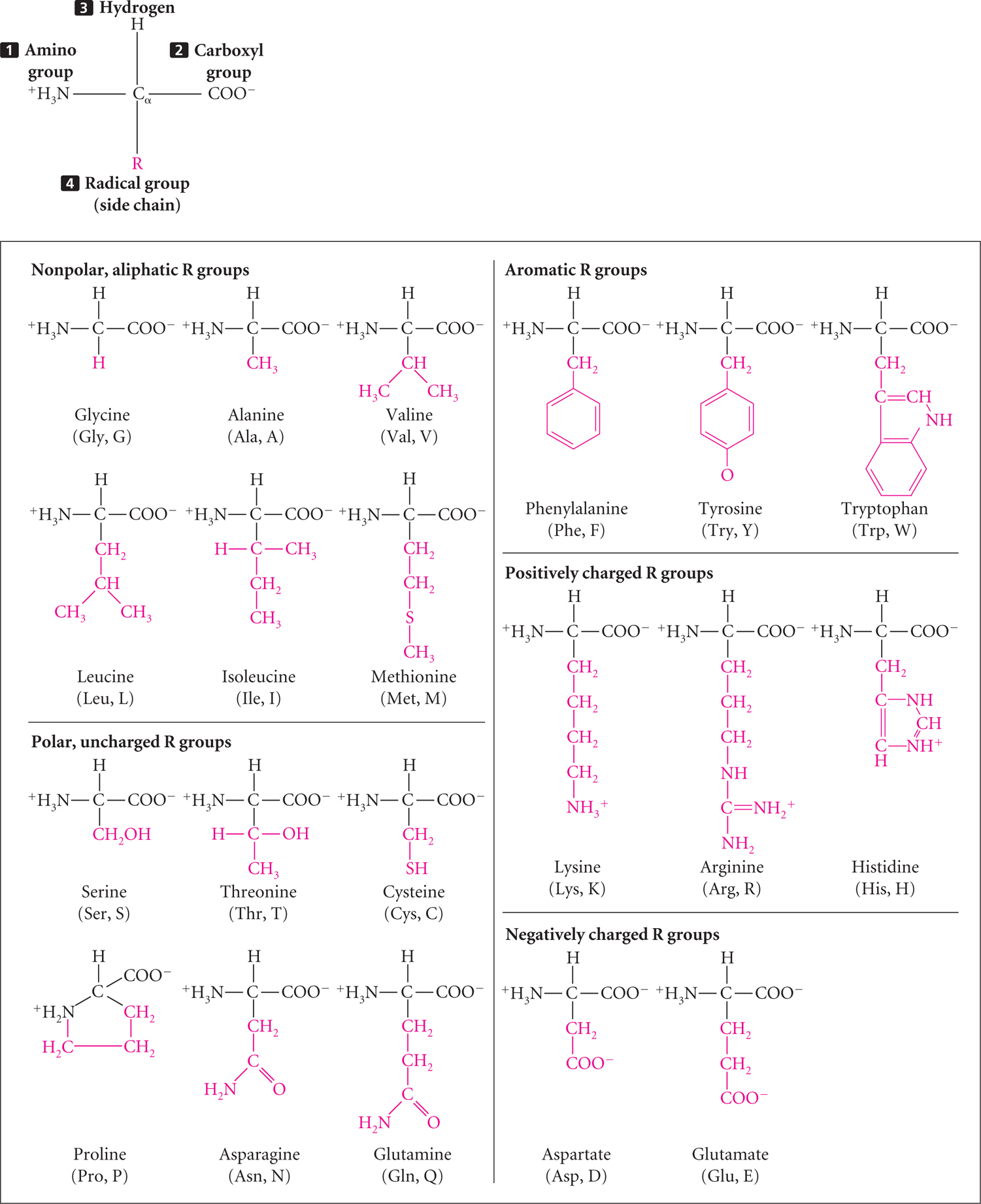
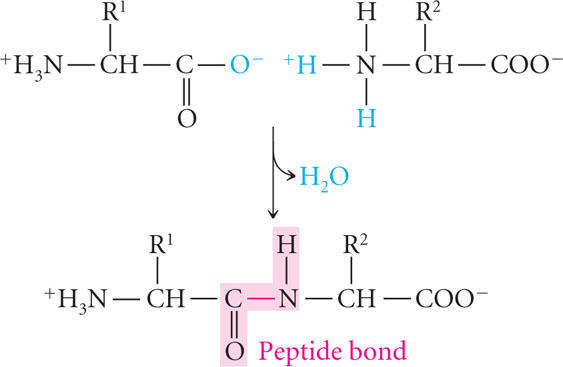
All proteins are composed of amino acids, linked end to end. Twenty common amino acids are found in proteins; these amino acids are shown in Figure 15.5 with both their three-letter and one-letter abbreviations (other amino acids sometimes found in proteins are modified forms of the common amino acids). The 20 common amino acids are similar in structure: each consists of a central carbon atom bonded to an amino group, a hydrogen atom, a carboxyl group, and an R (radical) group that differs for each amino acid. The amino acids in proteins are joined together by peptide bonds (Figure 15.6) to form polypeptide chains, and a protein consists of one or more polypeptide chains. Like nucleic acids, polypeptides have polarity, with one end having a free amino group (NH3+) and the other end possessing a free carboxyl group (COO−). Some proteins consist of only a few amino acids, whereas others may have thousands.
Protein Structure
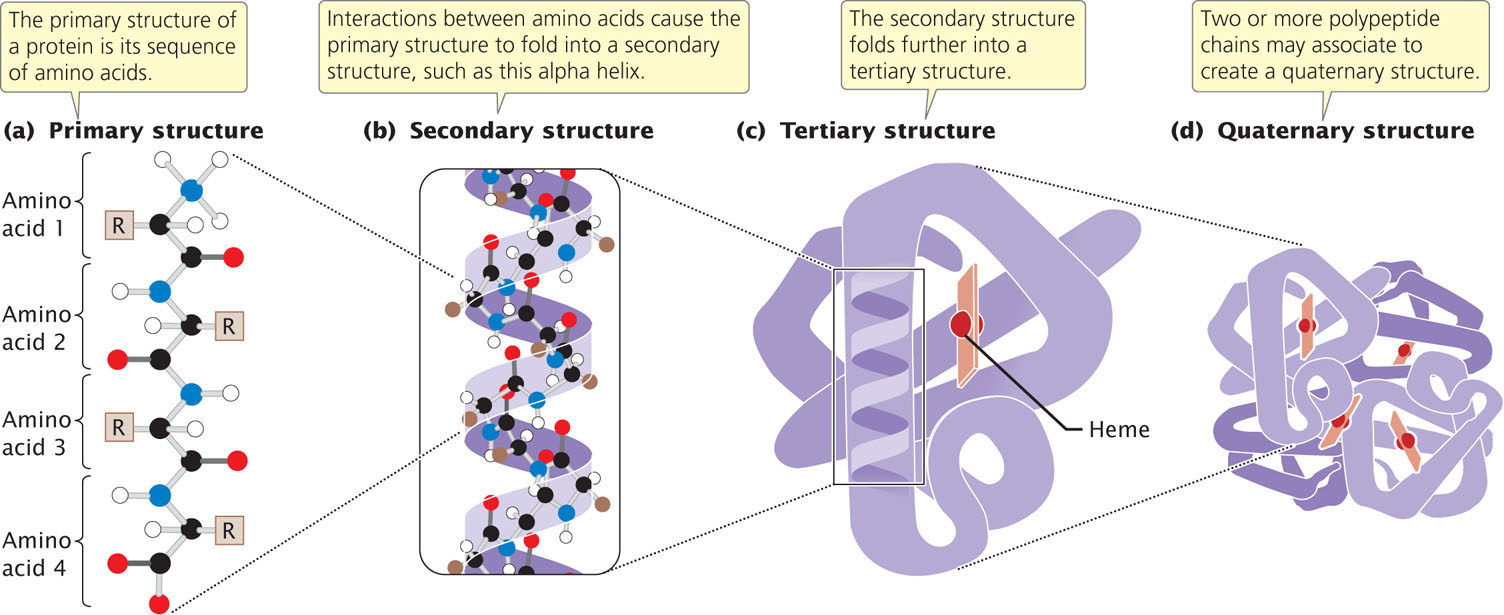
Like that of nucleic acids, the molecular structure of proteins has several levels of organization. The primary structure of a protein is its sequence of amino acids (Figure 15.7a). Through interactions between neighboring amino acids, a polypeptide chain folds and twists into a secondary structure (Figure 15.7b); two common secondary structures found in proteins are the beta (β) pleated sheet and the alpha (α) helix. Secondary structures interact and fold further to form a tertiary structure (Figure 15.7c), which is the overall, three-dimensional shape of the protein. The secondary and tertiary structures of a protein are largely determined by the primary structure—the amino acid sequence—of the protein. Finally, some proteins consist of two or more polypeptide chains that associate to produce a quaternary structure (Figure 15.7d).
416
417
CONCEPTS
The products of many genes are proteins whose actions produce the traits specified by these genes. Proteins are polymers consisting of amino acids linked by peptide bonds. The amino acid sequence of a protein is its primary structure. This structure folds to create the secondary and tertiary structures; two or more polypeptide chains may associate to create a quaternary structure.
 CONCEPT CHECK 2
CONCEPT CHECK 2
What primarily determines the secondary and tertiary structures of a protein?