15.3 Amino Acids Are Assembled into a Protein Through Translation
Now that we are familiar with the genetic code, we can begin to study how amino acids are assembled into proteins. Because more is known about translation in bacteria, we will focus primarily on bacterial translation. In most respects, eukaryotic translation is similar, although some significant differences will be noted.
Remember that only mRNAs are translated into proteins. Translation takes place on ribosomes; indeed, ribosomes can be thought of as moving protein-synthesizing machines. Through a variety of techniques, a detailed view of the structure of the ribosome has been produced in recent years, which has greatly improved our understanding of translation. A ribosome attaches near the 5′ end of an mRNA strand and moves toward the 3′ end, translating the codons as it goes (Figure 15.12). Synthesis begins at the amino end of the protein, and the protein is elongated by the addition of new amino acids to the carboxyl end. Protein synthesis includes a series of RNA–RNA interactions: interactions between the mRNA and the rRNA that hold the mRNA in the ribosome, between the codon on the mRNA and the anticodon on the tRNA, and between the tRNA and the rRNAs of the ribosome.
423
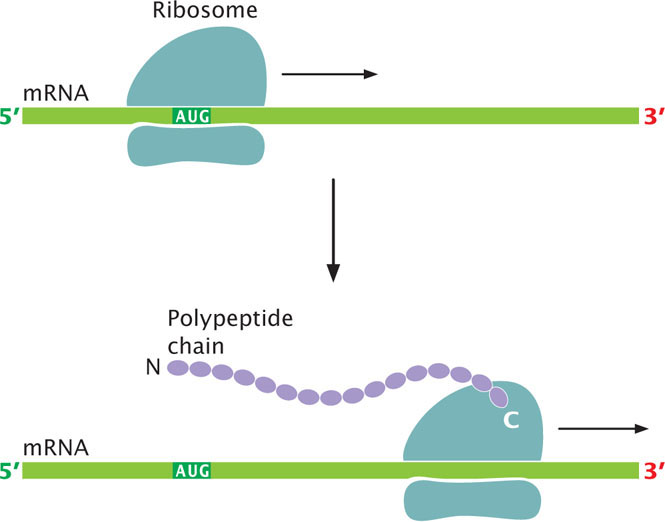
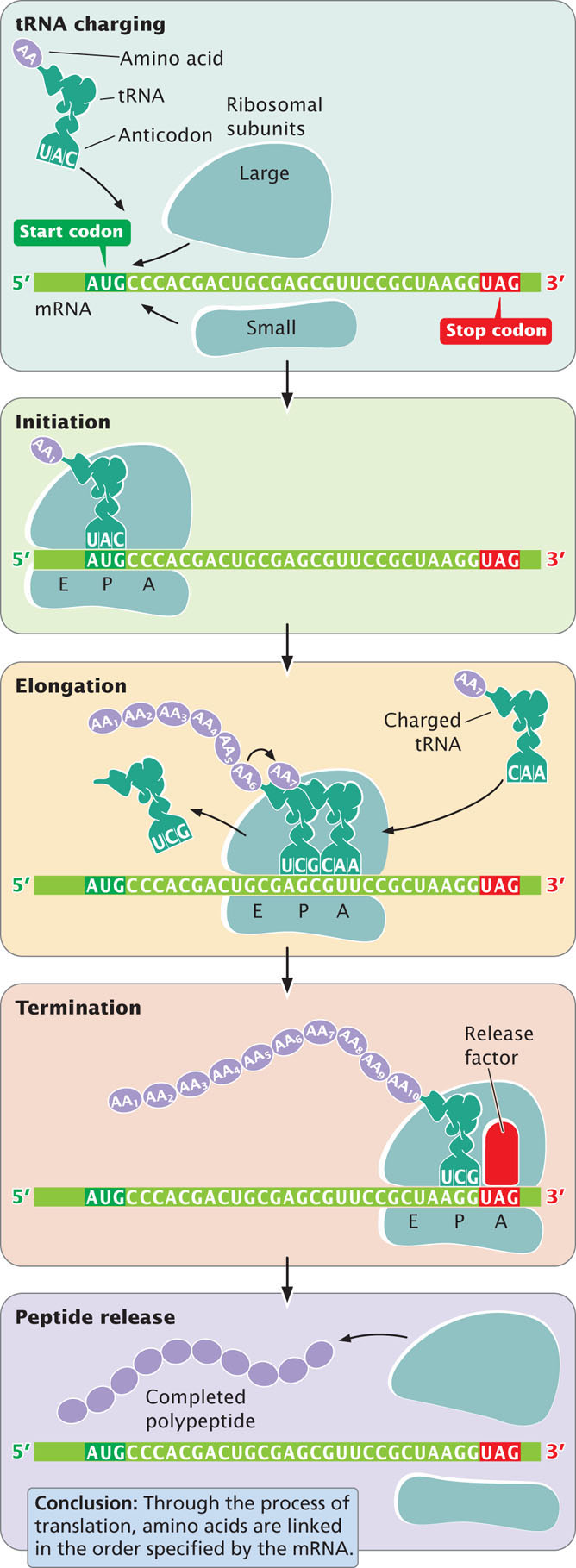
Protein synthesis can be conveniently divided into four stages: (1) tRNA charging, in which tRNAs bind to amino acids; (2) initiation, in which the components necessary for translation are assembled at the ribosome; (3) elongation, in which amino acids are joined, one at a time, to the growing polypeptide chain; and (4) termination, in which protein synthesis halts at the termination codon and the translation components are released from the ribosome.
The Binding of Amino Acids to Transfer RNAs
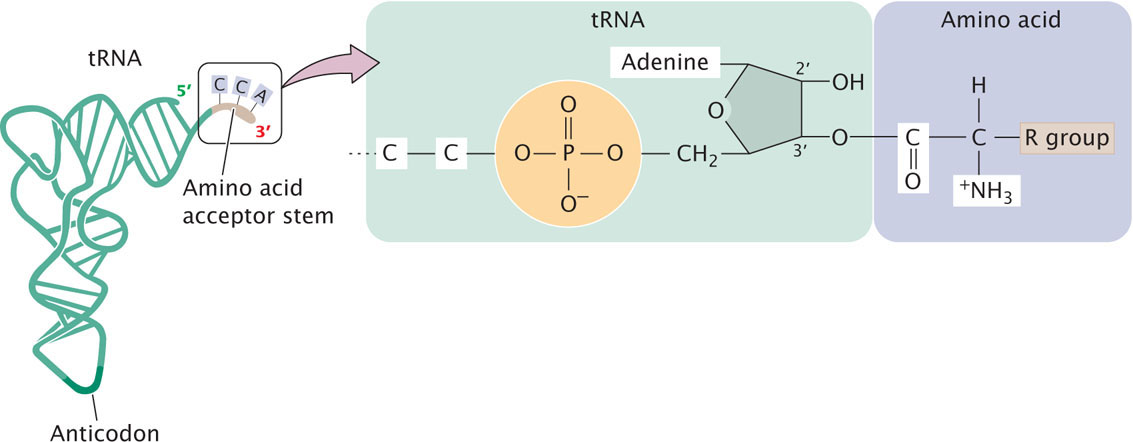
The first stage of translation is the binding of tRNA molecules to their appropriate amino acids, called tRNA charging. Each tRNA is specific for a particular amino acid. All tRNAs have the sequence CCA at the 3′ end, and the carboxyl group (COO−) of the amino acid is attached to the adenine nucleotide at the 3′ end of the tRNA (Figure 15.13). If each tRNA is specific for a particular amino acid but all amino acids are attached to the same nucleotide (A) at the 3′ end of a tRNA, how does a tRNA link up with its appropriate amino acid?
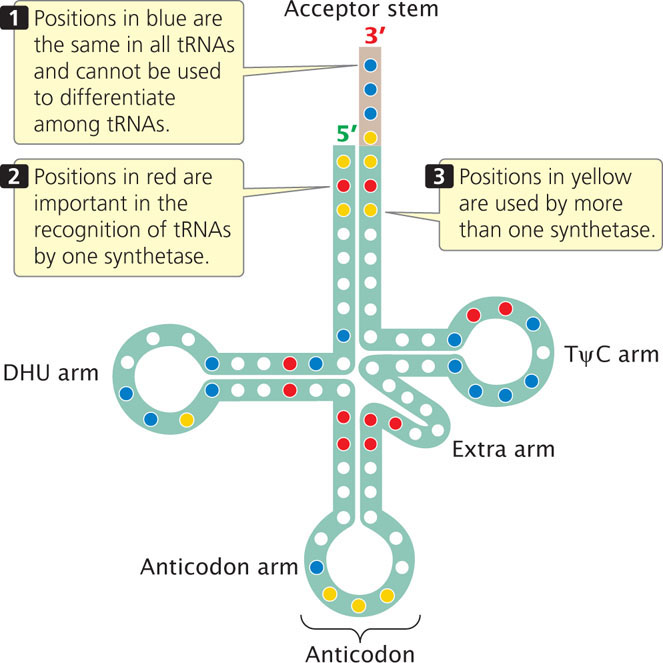
The key to specificity between an amino acid and its tRNA is a set of enzymes called aminoacyl-tRNA synthetases. A cell has 20 different aminoacyl-tRNA synthetases, one for each of the 20 amino acids. Each synthetase recognizes a particular amino acid, as well as all the tRNAs that accept that amino acid. Recognition of the appropriate amino acid by a synthetase is based on the different sizes, charges, and R groups of the amino acids. The recognition of tRNAs by a synthetase depends on the differing nucleotide sequences of the tRNAs. Researchers have identified which nucleotides are important in recognition by altering different nucleotides in a particular tRNA and determining whether the altered tRNA is still recognized by its synthetase (Figure 15.14).
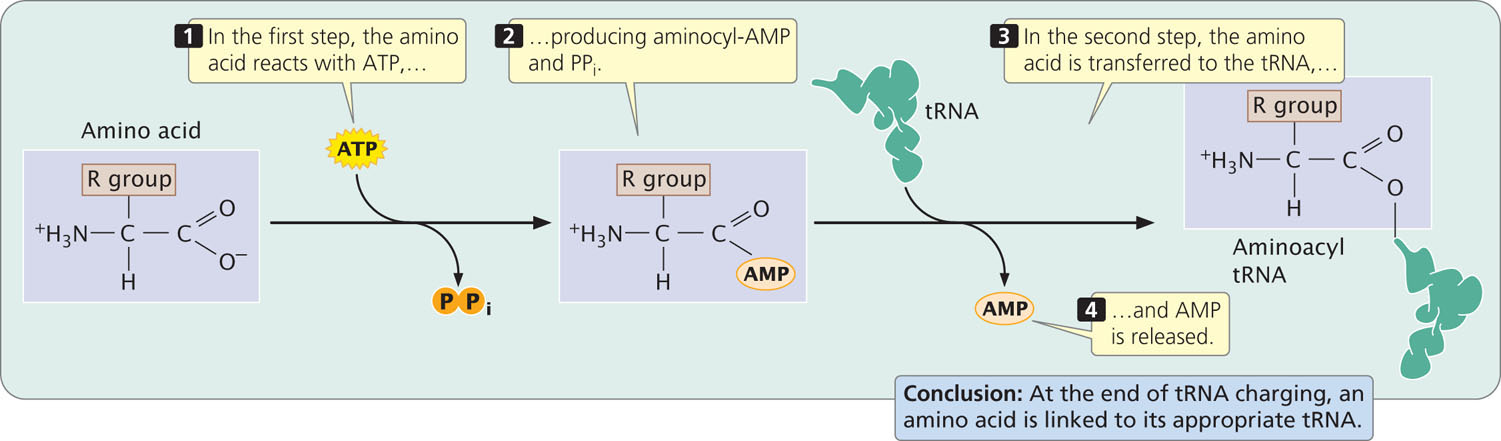
The attachment of a tRNA to its appropriate amino acid, termed tRNA charging, requires energy, which is supplied by adenosine triphosphate (ATP):
amino acid + tRNA + ATP → aminoacyl-tRNA + AMP + PPi
This reaction takes place in two steps (Figure 15.15). To identify the resulting aminoacylated tRNA, we write the three-letter abbreviation for the amino acid in front of the tRNA; for example, the amino acid alanine (Ala) attaches to its tRNA (tRNAAla), giving rise to its aminoacyl-tRNA (Ala-tRNAAla).
Errors in tRNA charging are rare: they occur in only about 1 in 10,000 to 1 in 100,000 reactions. This fidelity is due in part to the presence of editing (proofreading) activity in many of the synthetases. Editing activity detects and removes incorrectly paired amino acids from the tRNAs. Some antifungal chemical agents work by trapping tRNAs in the editing site of the enzyme, preventing their release and thus inhibiting the process of translation in the fungi.
CONCEPTS
Amino acids are attached to specific tRNAs by aminoacyl-tRNA synthetases in a two-step reaction that requires ATP.
 CONCEPT CHECK 6
CONCEPT CHECK 6
Amino acids bind to which part of the tRNA?
- anticodon
- DHU arm.
- 3′ End
- 5′ end
424
The Initiation of Translation
The second stage in the process of protein synthesis is initiation. At this stage, all the components necessary for protein synthesis assemble: (1) mRNA; (2) the small and large subunits of the ribosome; (3) a set of three proteins called initiation factors; (4) initiator tRNA with N-formylmethionine attached (fMet-tRNAfMet); and (5) guanosine triphosphate (GTP). Initiation comprises three major steps. First, mRNA binds to the small subunit of the ribosome. Second, initiator tRNA binds to the mRNA through base pairing between the codon and the anticodon. Third, the large ribosome joins the initiation complex. Let’s look at each of these steps more closely.
Initiation in Bacteria
The functional ribosome of bacteria exists as two subunits, the small 30S subunit and the large 50S subunit (Figure 15.16a). An mRNA molecule can bind to the small ribosome subunit only when the subunits are separate. Initiation factor 3 (IF-3) binds to the small subunit of the ribosome and prevents the large subunit from binding during initiation (Figure 15.16b). Another factor, initiation factor 1 (IF-1), enhances the disassociation of the large and small ribosomal subunits.
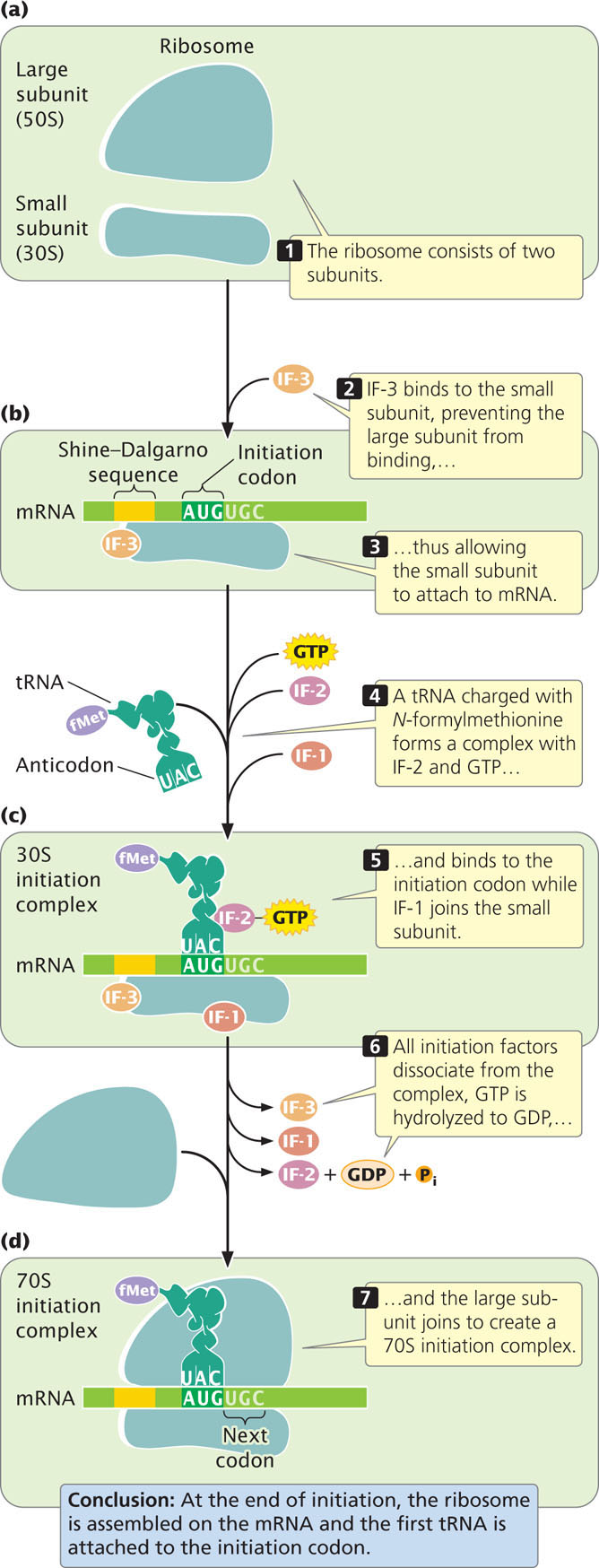
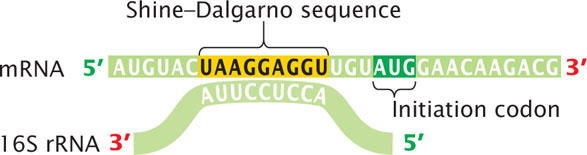
Where on the mRNA does the ribosome bind during initiation of translation? Key sequences on the mRNA required for ribosome binding have been identified in experiments designed to allow the ribosome to bind to mRNA but not proceed with protein synthesis; the ribosome is thereby stalled at the initiation site where binding occurs. A ribonuclease is added, which degrades all the mRNA except the region covered by the ribosome. The intact mRNA can be separated from the ribosome and studied. The sequence covered by the ribosome during initiation is from 30 to 40 nucleotides long and includes the AUG initiation codon. Within the ribosome-binding site is the Shine–Dalgarno consensus sequence (Figure 15.17; see also Chapter 14), which is complementary to a sequence of nucleotides at the 3′ end of 16S rRNA (part of the small subunit of the ribosome). During initiation, the nucleotides in the Shine–Dalgarno sequence pair with their complementary nucleotides in the 16S rRNA, allowing the small subunit of the ribosome to attach to the mRNA and positioning the ribosome directly over the initiation codon. These ribosome-binding sequences are within the 5′ untranslated region of the mRNA.
The initiator tRNA, fMet-tRNAfMet, attaches to the initiation codon (see Figure 15.16c). This requires initiation factor 2 (IF-2), which forms a complex with GTP.
At this point, the initiation complex consists of (1) the small subunit of the ribosome; (2) the mRNA; (3) the initiator tRNA with its amino acid (fMet-tRNAfMet); (4) one molecule of GTP; and (5) several initiation factors. These components are collectively known as the 30S initiation complex (see Figure 15.16c). In the final step of initiation, IF-3 dissociates from the small subunit, allowing the large subunit of the ribosome to join the initiation complex. The molecule of GTP (provided by IF-2) is hydrolyzed to guanosine diphosphate (GDP), and the initiation factors dissociate (see Figure 15.16d). When the large subunit has joined the initiation complex, the complex is called the 70S initiation complex.
425
Initiation in Eukaryotes
Similar events take place in the initiation of translation in eukaryotic cells, but there are some important differences. In bacterial cells, sequences in 16S rRNA of the small subunit of the ribosome bind to the Shine–Dalgarno sequence in mRNA. No analogous consensus sequence exists in eukaryotic mRNA. Instead, the cap at the 5′ end of eukaryotic mRNA plays a critical role in the initiation of translation. In a series of steps, the small subunit of the eukaryotic ribosome, initiation factors, and the initiator tRNA with its amino acid (Met-tRNAiMet) form an initiation complex that recognizes the cap and binds there. The initiation complex then moves along (scans) the mRNA until it locates the first AUG codon. The identification of the start codon is facilitated by the presence of a consensus sequence (called the Kozak sequence) that surrounds the start codon:
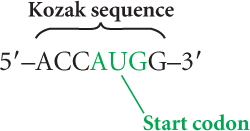
Another important difference is that eukaryotic initiation requires at least seven initiation factors. Some factors keep the ribosomal subunits separated, just as IF-3 does in bacterial cells. Others recognize the 5′ cap on mRNA and allow the small subunit of the ribosome to bind there. Still others possess RNA helicase activity, which is used to unwind secondary structures that may exist in the 5′ untranslated region of mRNA, allowing the small subunit to move down the mRNA until the initiation codon is reached. Other initiation factors help bring Met-tRNAiMet to the initiation complex.
In eukaryotes, the cap is initially bound by several proteins, one of which is the cap-binding complex (CBC). The CBC aids in exporting the mRNA from the nucleus and then promotes the “pioneer” or initial round of translation in the cytoplasm. This first round of translation plays an important role in checking for errors in the mRNA (see Messenger RNA Surveillance in the next section). After the pioneer round of translation, the CBC is replaced by eukaryotic initiation factor 4E (eIF-4E), which promotes continued translation of the mRNA.
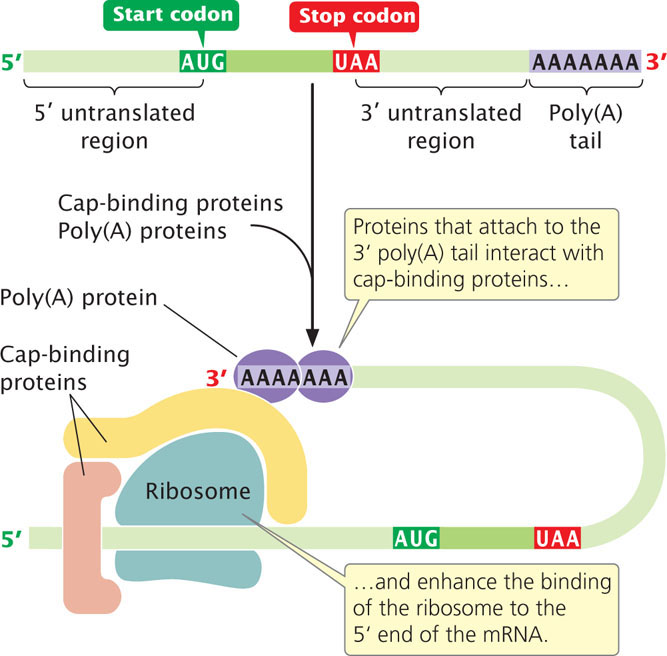
The poly(A) tail at the 3′ end of eukaryotic mRNA also plays a role in the initiation of translation. During initiation, proteins that attach to the poly(A) tail interact with proteins that bind to the 5′ cap, enhancing the binding of the small subunit of the ribosome to the 5′ end of the mRNA. This interaction indicates that the 3′ end of mRNA bends over and associates with the 5′ cap during the initiation of translation, forming a circular structure known as the closed loo (Figure 15.18). A few eukaryotic mRNAs contain internal ribosome entry sites, where ribosomes can bind directly without first attaching to the 5′ cap.
426
CONCEPTS
In the initiation of translation in bacterial cells, the small ribosomal subunit attaches to mRNA, and initiator tRNA attaches to the initiation codon. This process requires several initiation factors (IF-1, IF-2, and IF-3) and GTP. In the final step, the large ribosomal subunit joins the initiation complex.
 CONCEPT CHECK 7
CONCEPT CHECK 7
During the initiation of translation, the small ribosome binds to which consensus sequence in bacteria?
Elongation
The next stage in protein synthesis is elongation, in which amino acids are joined to create a polypeptide chain. Elongation requires (1) the 70S complex just described; (2) tRNAs charged with their amino acids; (3) several elongation factors; and (4) GTP.
A ribosome has three sites that can be occupied by tRNAs; the aminoacyl or A site, the peptidyl or P site, and the exit or E site (Figure 15.19a). The initiator tRNA immediately occupies the P site (the only site to which the fMet-tRNAfMet is able to bind), but all other tRNAs first enter the A site. At the end of initiation, the ribosome is attached to the mRNA, and fMet-tRNAfMet is positioned over the AUG start codon in the P site; the adjacent A site is unoccupied (see Figure 15.19a).
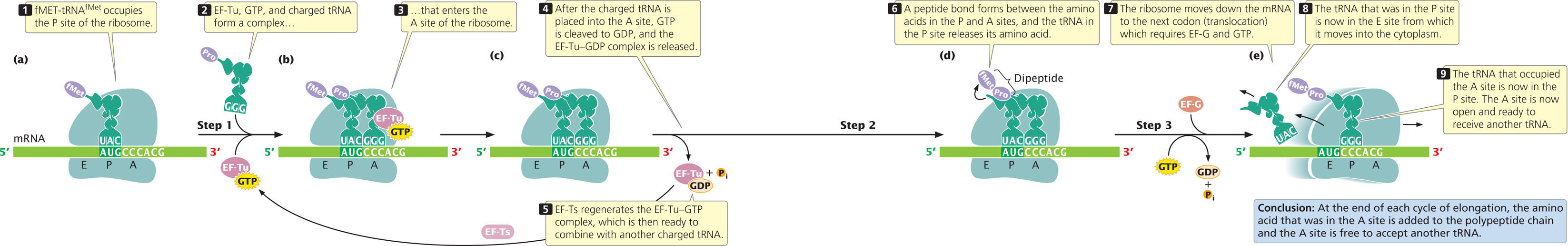
Elongation takes place in three steps. In the first step (Figure 15.19b), a charged tRNA binds to the A site. This binding takes place when elongation factor Tu (EF-Tu) joins with GTP and then with a charged tRNA to form a three-part complex. This complex enters the A site of the ribosome, where the anticodon on the tRNA pairs with the codon on the mRNA. After the charged tRNA is in the A site, GTP is cleaved to GDP, and the EF-Tu–GDP complex is released (Figure 15.19c). Elongation factor Ts (EF-Ts) regenerates EF-Tu–GDP to EF-Tu–GTP. In eukaryotic cells, a similar set of reactions delivers the charged tRNA to the A site.
The second step of elongation is the formation of a peptide bond between the amino acids that are attached to tRNAs in the P and A sites (Figure 15.19d). The formation of this peptide bond releases the amino acid in the P site from its tRNA. Peptide bond formation occurs within the peptidyl transferase center, which is part of the large subunit of the ribosome. Evidence indicates that the catalytic activity is a property of ribosomal RNA in the large subunit of the ribosome (the 23S rRNA in bacteria, the 28S RNA in eukaryotes); this rRNA acts as a ribozyme.
The third step in elongation is translocation (Figure 15.19e), the movement of the ribosome down the mRNA in the 5′→3′ direction. This step positions the ribosome over the next codon and requires elongation factor G (EF-G) and the hydrolysis of GTP to GDP. Because the tRNAs in the P and A sites are still attached to the mRNA through codon–anticodon pairing, they do not move with the ribosome as it translocates. Consequently, the ribosome shifts so that the tRNA that previously occupied the P site now occupies the E site, from which it moves into the cytoplasm where it can be recharged with another amino acid. Translocation also causes the tRNA that occupied the A site (which is attached to the growing polypeptide chain) to be in the P site, leaving the A site open. Thus, the progress of each tRNA through the ribosome in the course of elongation can be summarized as follows: cytoplasm → A site → P site → E site → cytoplasm. As stated earlier, the initiator tRNA is an exception: it attaches directly to the P site and never occupies the A site.
427
After translocation, the A site of the ribosome is empty and ready to receive the tRNA specified by the next codon. The elongation cycle (see Figure 15.19b through e) repeats itself: a charged tRNA and its amino acid occupy the A site, a peptide bond is formed between the amino acids in the A and P sites, and the ribosome translocates to the next codon. Throughout the cycle, the polypeptide chain remains attached to the tRNA in the P site. Another protein called translational elongation factor P (EF-P), enhances the translation of proteins that contain consecutive copies of the amino acid proline. If EF-P is absent, ribosomes often stall during the translation of these polyproline-containing proteins.
Messenger RNAs, although single stranded, often contain secondary structures formed by pairing of complementary bases on different parts of the mRNA (see Figure 13.1b). As the ribosome moves along the mRNA, these secondary structures are unwound by helicase activity located in the small subunit of the ribosome.
Recently, researchers have developed methods for following a single ribosome as it translates individual codons of an mRNA molecule. These studies revealed that translation does not take place in a smooth continuous fashion. Each translocation step typically requires less than a tenth of a second, but sometimes there are distinct pauses, often lasting a few seconds, between each translocation event when the ribosome moves from one codon to another. Thus, translation takes place in a series of quick translocations interrupted by brief pauses. In addition to the short pauses between translocation events, translation may be interrupted by longer pauses—lasting from 1 to 2 minutes—that may play a role in regulating the process of translation.
Elongation in eukaryotic cells takes place in a similar manner. Eukaryotes possess at least three elongation factors, one of which also acts in initiation and termination. Another of the elongation factors used in eukaryotes, called eukaryotic elongation factor 2 (eEF-2), is the target of a toxin produced by bacteria that causes diphtheria, a disease that until recently was a leading killer of children. The diphtheria toxin inhibits eEF-2, preventing the translocation of the ribosome along the mRNA, and protein synthesis ceases.
CONCEPTS
Elongation consists of three steps: (1) a charged tRNA enters the A site, (2) a peptide bond is created between amino acids in the A and P sites, and (3) the ribosome translocates to the next codon. Elongation requires several elongation factors and GTP.
 CONCEPT CHECK 8
CONCEPT CHECK 8
In elongation, the creation of peptide bonds between amino acids is catalyzed by
- rRNA.
- protein in the small subunit.
- protein in the large subunit.
- tRNA.
Termination
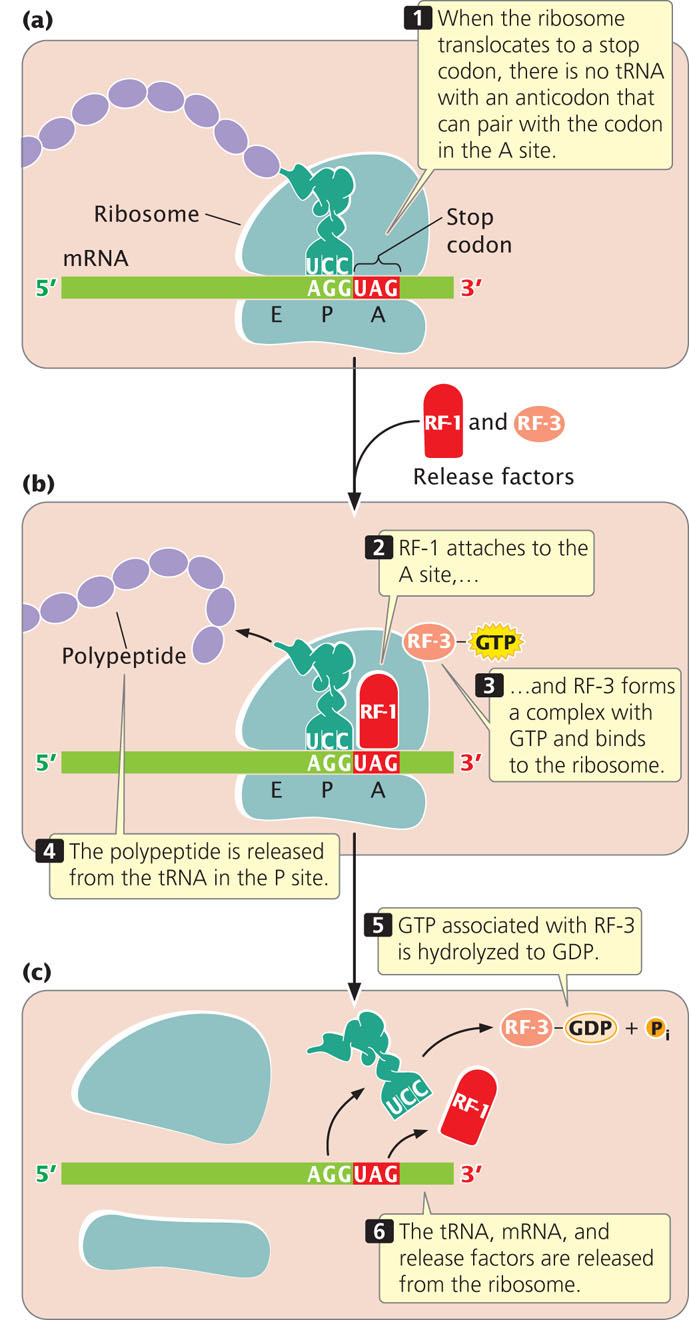
Protein synthesis terminates when the ribosome translocates to a termination codon. Because there are no tRNAs with anticodons complementary to the termination codons, no tRNA enters the A site of the ribosome when a termination codon is encountered (Figure 15.20a). Instead, proteins called release factors bind to the ribosome (Figure 15.20b). Escherichia coli has three release factors—RF-1, RF-2, and RF-3. Release factor 1 binds to the termination codons UAA and UAG, and RF-2 binds to UGA and UAA. The binding of release factor RF-1 or RF-2 to the A site of the ribosome promotes the cleavage of the tRNA in the P site from the polypeptide chain and the release of the polypeptide. Release factor 3 binds to the ribosome and forms a complex with GTP. This complex brings about a conformational change in the ribosome, releasing RF-1 or RF-2 from the A site and causing the tRNA in the P site to move to the E site; in the process, GTP is hydrolyzed to GDP. Additional factors help bring about the release of the tRNA from the P site, the release of the mRNA from the ribosome, and the dissociation of the ribosome (Figure 15.20c). It is important to note that the termination codon is not located at the 3′ end of the mRNA; rather, the termination codon is followed by a number of nucleotides that constitute the 3′ untranslated region (UTR) of the mRNA. The 3′ UTR often contains sequences that affect the stability of the mRNA and influence whether translation takes place (see Chapter 17).
428
429
Recent research shows that some bacterial ribosomes engage in a type of proofreading, similar to the way that DNA polymerases proofread during replication. After translocation, the ribosome checks the interaction between the mRNA and the tRNA in the P site. If the wrong tRNA was added, the alignment between the mRNA and tRNA will be incorrect, which triggers premature termination of translation. Evidence suggests that an important function of RF-3 is to help bring about termination of translation when the wrong tRNA has been used. Explore the process of bacterial translation by examining the consequences of various mutations in the coding region of a gene in  Animation 15.1.
Animation 15.1.
Translation in eukaryotic cells terminates in a similar way, except that there are two release factors: eRF-1, which recognizes all three termination codons, and eRF-2, which binds GTP and stimulates the release of the polypeptide from the ribosome. No translational proofreading, such as that stimulated by RF-3 in bacteria, has been observed eukaryotic cells.  TRY PROBLEM 30 AND 34
TRY PROBLEM 30 AND 34
CONCEPTS
Termination takes place when the ribosome reaches a termination codon. Release factors bind to the termination codon, causing the release of the polypeptide from the last tRNA, of the tRNA from the ribosome, and of the mRNA from the ribosome.
The overall process of protein synthesis, including tRNA charging, initiation, elongation, and termination, is summarized in Figure 15.21. The components taking part in this process are listed in Table 15.4.
| Stage | Component | Function |
|---|---|---|
| tRNA charging | Amino acids | Building blocks of proteins |
| tRNAs | Deliver amino acids to ribosomes | |
| Aminoacyl-tRNA synthetases | Attaches amino acids to tRNAs | |
| ATP | Provides energy for binding amino acid to tRNA | |
| Initiation | mRNA | Carries coding instructions |
| fMet-tRNAfMet | Provides first amino acid in peptide | |
| 30S ribosomal subunit | Attaches to mRNA | |
| 50S ribosomal subunit | Stabilizes tRNAs and amino acids | |
| Initiation factor 1 | Enhances dissociation of large and small subunits of ribosome | |
| Initiation factor 2 | Binds GTP; delivers fMet-tRNAfMet to initiation codon | |
| Initiation factor 3 | Binds to 30S subunit and prevents association with 50S subunit | |
| Elongation | 70S initiation complex | Functional ribosome with A, P, and E sites where protein synthesis takes place |
| Charged tRNAs | Bring amino acids to ribosome and help assemble them in order specified by mRNA | |
| Elongation factor Tu | Binds GTP and charged tRNA; delivers charged tRNA to A site | |
| Elongation factor Ts | Generates active elongation factor Tu | |
| Elongation factor G | Stimulates movement of ribosome to next codon | |
| GTP | Provides energy | |
| Peptidyl transferase center | Creates peptide bond between amino acids in A site and P site | |
| Termination | Release factors 1, 2, and 3 | Bind to ribosome when stop codon is reached and terminate translation |
430
CONNECTING CONCEPTS: A Comparison of Bacterial and Eukaryotic Translation
We have now considered the process of translation in bacterial cells and noted some distinctive differences that exist in eukaryotic cells. Let’s reflect on some of the important similarities and differences of protein synthesis in bacterial and eukaryotic cells.
First, we should emphasize that the genetic code of bacterial and eukaryotic cells is virtually identical; the only difference is in the amino acid specified by the initiation codon. In bacterial cells, the initiation AUG encodes a modified type of methionine, N-formylmethionine, whereas, in eukaryotic cells, initiation AUG encodes unformylated methionine. One consequence of the fact that bacteria and eukaryotes use the same code is that eukaryotic genes can be translated in bacterial systems, and vice versa; this feature makes genetic engineering possible, as we will see in Chapter 19.
Another difference is that transcription and translation take place simultaneously in bacterial cells, but the nuclear envelope separates these processes in eukaryotic cells. The physical separation of transcription and translation has important implications for the control of gene expression, which we will consider in Chapter 17, and it allows for extensive modification of eukaryotic mRNAs, as discussed in Chapter 14.
Yet another difference is that mRNA in bacterial cells is shortlived, typically lasting only a few minutes, but mRNA in eukaryotic cells can last hours or days. The 5′ cap and 3′ poly(A) tail found on eukaryotic mRNAs add to their stability (see Chapter 14).
In both bacterial and eukaryotic cells, aminoacyl-tRNA synthetases attach amino acids to their appropriate tRNAs and the chemical process is the same. There are significant differences in the sizes and compositions of bacterial and eukaryotic ribosomal subunits. For example, the large subunit of the eukaryotic ribosome contains three rRNAs, whereas the bacterial ribosome contains only two. These differences allow antibiotics and other substances to inhibit bacterial translation while having no effect on the translation of eukaryotic nuclear genes, as will be discussed later in this chapter.
Other fundamental differences lie in the process of initiation. In bacterial cells, the small subunit of the ribosome attaches directly to the region surrounding the start codon through hydrogen bonding between the Shine–Dalgarno consensus sequence in the 5′ untranslated region of the mRNA and a sequence at the 3′ end of the 16S rRNA. In contrast, the small subunit of a eukaryotic ribosome first binds to proteins attached to the 5′ cap on mRNA and then migrates down the mRNA, scanning the sequence until it encounters the first AUG initiation codon. Additionally, a larger number of initiation factors take part in eukaryotic initiation than in bacterial initiation.
Elongation and termination are similar in bacterial and eukaryotic cells, although different elongation and termination factors are used. In both types of organisms, mRNAs are translated multiple times and are simultaneously attached to several ribosomes, forming polyribosomes, as discussed in Section 15.4.
Much less is known about the process of translation in archaea, but they appear to possess a mixture of eubacterial and eukaryotic features. Because archaea lack nuclear membranes, transcription and translation take place simultaneously, just as they do in eubacterial cells. Archaea utilize unformylated methionine as the initiator amino acid, a characteristic of eukaryotic translation. Some of the initiation and release factors in archaea are similar to those found in eubacteria, whereas others are similar to those found in eukaryotes. Finally, some of the antibiotics that inhibit translation in eubacteria have no effect on translation in archaea, providing further evidence of the fundamental differences between eubacteria and archaea.