17.3 The Initiation of Transcription Is Regulated by Transcription Factors and Regulator Proteins
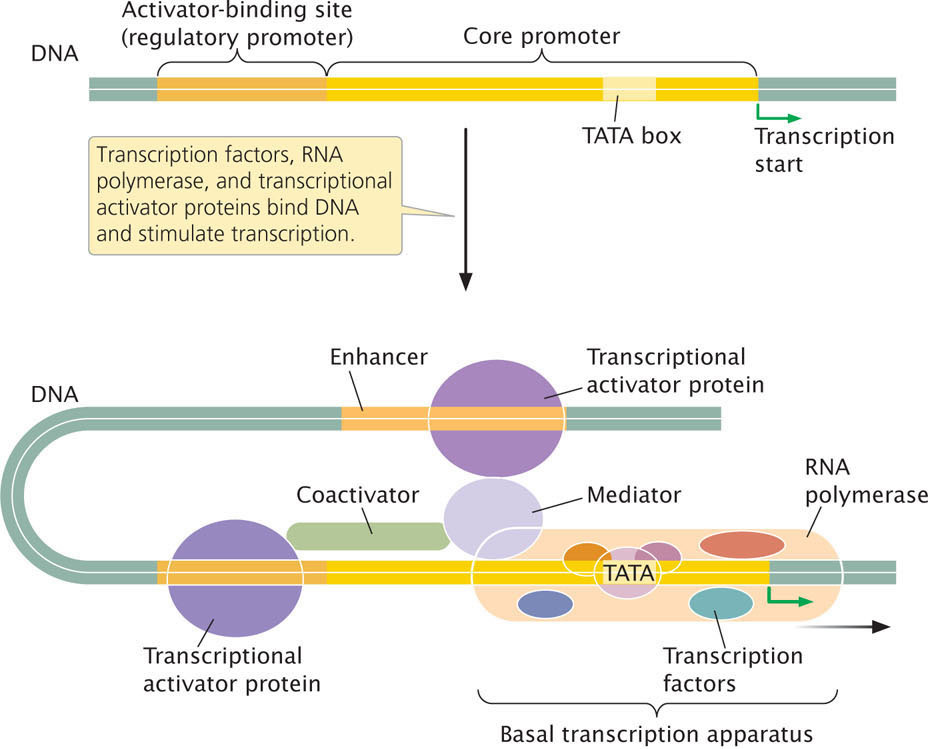
We just considered one level at which gene expression is controlled—the alteration of chromatin and DNA structure. We now turn to another important level of control—control through the binding of proteins to DNA sequences that affect transcription. Transcription is an important level of control in eukaryotic cells, and this control requires a number of different types of proteins and regulatory elements. The initiation of eukaryotic transcription was discussed in detail in Chapter 13. Recall that general transcription factors and RNA polymerase assemble into a basal transcription apparatus, the complex of RNA polymerase, transcription factors, and other proteins that carry out transcription. The basal transcription apparatus binds to a core promoter located immediately upstream of a gene and is capable of minimal levels of transcription; transcriptional regulator proteins are required to bring about normal levels of transcription. These proteins bind to a regulatory promoter, which is located upstream of the core promoter (Figure 17.5), and to enhancers, which may be located some distance from the gene. Some transcriptional regulator proteins are activators, stimulating transcription. Others are represssors, inhibiting transcription.
Transcriptional Activators and Coactivators
Transcriptional activator proteins stimulate and stabilize the basal transcription apparatus at the core promoter. The activators may interact directly with the basal transcription apparatus or indirectly through protein coactivators. Some activators and coactivators, as well as the general transcription factors, also have acetyltransferase activity and so further stimulate transcription by altering chromatin structure.
Transcriptional activator proteins have two distinct functions (see Figure 17.5). First, they are capable of binding DNA at a specific base sequence, usually a consensus sequence in a regulatory promoter or enhancer; for this function, most transcriptional activator proteins contain one or more DNA-binding motifs, such as the helix-turn-helix, zinc finger, and leucine zipper (see Chapter 16). A second function is the ability to interact with other components of the transcriptional apparatus and influence the rate of transcription.
Within the regulatory promoter are typically several different consensus sequences to which different transcriptional activators can bind. Among different promoters, activator-binding sites are mixed and matched in different combinations (Figure 17.6), and so each promoter is regulated by a unique combination of transcriptional activator proteins.
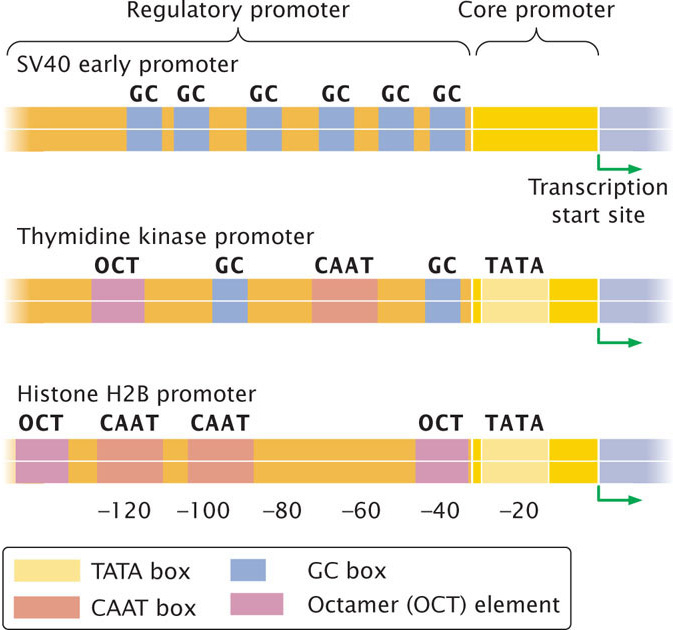
Transcriptional activator proteins bind to the consensus sequences in the regulatory promoter and affect the assembly or stability of the basal transcription apparatus at the core promoter. One of the components of the basal transcription apparatus is a complex of proteins called the mediator (see Figure 17.6). Transcriptional activator proteins binding to sequences in the regulatory promoter (or enhancer, see next section) make contact with the mediator and affect the rate at which transcription is initiated. Some regulatory promoters also contain sequences that are bound by proteins that lower the rate of transcription through inhibitory interactions with the mediator.
480
Regulation of Galactose Metabolism Through GAL4
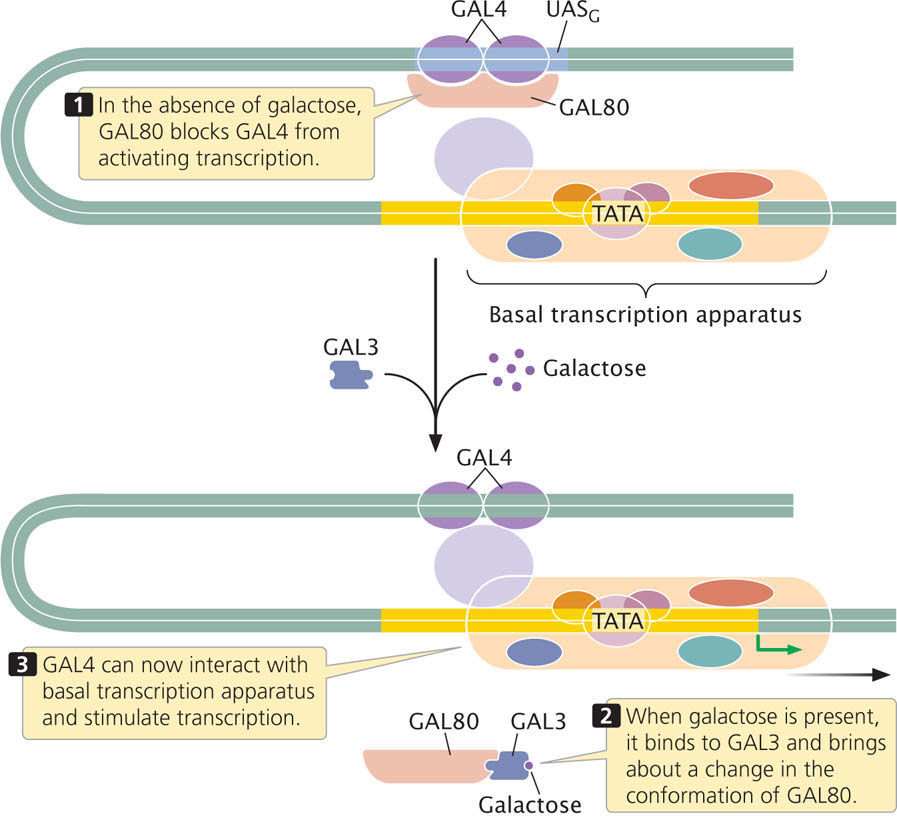
An example of a transcriptional activator protein is GAL4, which regulates the transcription of several yeast genes whose products metabolize galactose. Like the genes in the lac operon, the genes that control galactose metabolism are inducible: when galactose is absent, these genes are not transcribed and the proteins that break down galactose are not produced; when galactose is present, the genes are transcribed and the enzymes are synthesized. GAL4 contains several zinc fingers and binds to a DNA sequence called UASG (upstream activating sequence for GAL4). UASG exhibits the properties of an enhancer—a regulatory sequence that may be some distance from the regulated gene and is independent of the gene in position and orientation (see Chapter 13). When bound to UASG, GAL4 activates the transcription of yeast genes needed for metabolizing galactose. GAL4 and a number of other transcriptional activator proteins contain multiple amino acids with negative charges that form an acidic activation domain. These acidic activators stimulate transcription by enhancing the assembly of the basal transcription apparatus.
A particular region of GAL4 binds another protein called GAL80, which regulates the activity of GAL4 in the presence of galactose. When galactose is absent, GAL80 binds to GAL4, preventing GAL4 from activating transcription (Figure 17.7). When galactose is present, however, it binds to another protein called GAL3, which interacts with GAL80, causing a conformational change in GAL80 so that it can no longer bind GAL4. The GAL4 protein is then free to activate the transcription of the genes, whose products metabolize galactose.
481
Transcriptional Repressors
Some regulatory proteins in eukaryotic cells act as repressors, inhibiting transcription. These repressors bind to sequences in the regulatory promoter or to distant sequences called silencers, which, like enhancers, are position and orientation independent. Unlike repressors in bacteria, most eukaryotic repressors do not directly block RNA polymerase. These repressors may compete with activators for DNA binding sites: when a site is occupied by an activator, transcription is activated, but, if a repressor occupies that site, there is no activation. Alternatively, a repressor may bind to sites near an activator site and prevent the activator from contacting the basal transcription apparatus. A third possible mechanism of repressor action is direct interference with the assembly of the basal transcription apparatus, thereby blocking the initiation of transcription.
CONCEPTS
Transcriptional regulatory proteins in eukaryotic cells can influence the initiation of transcription by affecting the stability or assembly of the basal transcription apparatus. Some regulatory proteins are activators and stimulate transcription; others are repressors and inhibit the initiation of transcription.
 CONCEPT CHECK 2
CONCEPT CHECK 2
Most transcriptional activator proteins affect transcription by interacting with
- introns.
- the basal transcription apparatus.
- DNA polymerase.
- the terminator.
Enhancers and Insulators
Enhancers are capable of affecting transcription at distant promoters. For example, an enhancer that regulates the gene encoding the alpha chain of the T-cell receptor is located 69,000 bp downstream of the gene’s promoter. Furthermore, the exact position and orientation of an enhancer relative to the promoter can vary. How can an enhancer affect the initiation of transcription taking place at a promoter that is tens of thousands of base pairs away? In many cases, regulator proteins bind to the enhancer and cause the DNA between the enhancer and the promoter to loop out, bringing the promoter and enhancer close to each other, and so the transcriptional regulator proteins are able to directly interact with the basal transcription apparatus at the core promoter (see Figure 17.5). Some enhancers may be attracted to promoters by proteins that bind to sequences in the regulatory promoter and “tether” the enhancer close to the core promoter. Enhancers may also affect transcription by undergoing modifications that alter chromatin structure. A typical enhancer is about 500 bp in length and contains 10 binding sites for proteins that regulate transcription.
Recent research demonstrates that many enhancers are themselves transcribed into short RNA molecules called enhancer RNAs (eRNAs). Evidence suggests that transcription of an enhancer is often associated with transcription at the promoters that the enhancers affect. How transcription at the enhancer might affect transcription occurring at a distant promoter is not clear. Enhancers might recruit RNA polymerase, which is then transferred to the promoter when the enhancer interacts with the promoter. Alternatively, transcription of the enhancer might allow the chromatin to adopt a more open configuration, which then facilitates transcription at nearby promoters.
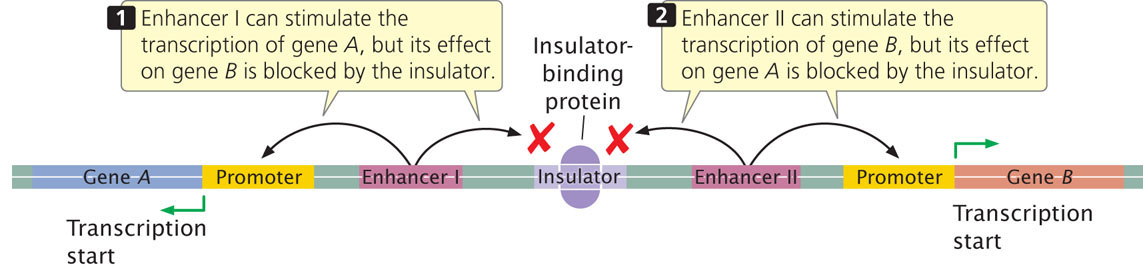
Most enhancers are capable of stimulating any promoter in their vicinities. Their effects are limited, however, by insulators (also called boundary elements), which are DNA sequences that block or insulate the effect of enhancers in a position-dependent manner. If the insulator lies between the enhancer and the promoter, it blocks the action of the enhancer; but, if the insulator lies outside the region between the two, it has no effect (Figure 17.8). Specific proteins bind to insulators and play a role in their blocking activity. Some insulators also limit the spread of changes in chromatin structure that affect transcription. Some enhancer-like elements are found in prokaryotes.  TRY PROBLEM 23
TRY PROBLEM 23
CONCEPTS
Some regulatory proteins bind to enhancers, which are regulatory elements that are distant from the gene whose transcription they stimulate. Insulators are DNA sequences that block the action of enhancers.
 CONCEPT CHECK 3
CONCEPT CHECK 3
How does the binding of regulatory proteins to enhancers affect transcription at genes that are thousands of base pairs away?
482
Regulation of Transcriptional Stalling and Elongation
Transcription in eukaryotes is often regulated through factors that affect the initiation of transcription, including changes in chromatin structure, transcription factors, and transcriptional regulatory proteins. Research indicates that transcription may also be controlled through factors that affect stalling and elongation of RNA polymerase after transcription has been initiated.
The basal transcription apparatus—consisting of RNA polymerase, transcription factors, and other proteins—assembles at the core promoter. When the initiation of transcription has taken place, RNA polymerase moves downstream, transcribing the structural gene and producing an RNA product. At some genes, RNA polymerase initiates transcription and transcribes from 24 to 50 nucleotides of RNA but then pauses or stalls. For example, stalling is observed at genes that encode heat-shock proteins in Drosophila—proteins that help to prevent damage from stressing agents such as extreme heat. Heat-shock proteins are produced by a large number of different genes. During times of environmental stress, the transcription of all the heat-shock genes is greatly elevated. RNA polymerase initiates transcription at heat-shock genes in Drosophila but, in the absence of stress, stalls downstream of the transcription initiation site. Stalled polymerases are released when stress is encountered, allowing rapid transcription of the genes and the production of heat-shock proteins that facilitate adaptation to the stressful environment.
Stalling was formerly thought to take place at only a small number of genes, but research now indicates that stalling is widespread throughout eukaryotic genomes. For example, stalled RNA polymerases were found at hundreds of genes in Drosophila. Several factors that promote stalling have been identified; one of these is a protein called negative elongation factor (NELF), which binds to RNA polymerase and causes it to stall after initiation. Another protein called positive transcription elongation factor b (P-TEFb) relieves stalling and promotes elongation by phosphorylating NELF and RNA polymerase, perhaps by causing NELF to dissociate from the polymerase.
CONCEPTS
At some genes, RNA polymerase may pause or stall downstream of the promoter. Regulatory factors affect stalling and the elongation of transcription.
Coordinated Gene Regulation
Although most eukaryotic cells do not possess operons, several eukaryotic genes may be activated by the same stimulus. Groups of bacterial genes are often coordinately expressed (turned on and off together) because they are physically clustered as an operon and have the same promoter, but coordinately expressed genes in eukaryotic cells are not clustered. How, then, is the transcription of eukaryotic genes coordinately controlled if they are not organized into an operon?
Genes that are coordinately expressed in eukaryotic cells are able to respond to the same stimulus because they have short regulatory sequences in common in their promoters or enhancers. For example, different eukaryotic heat-shock genes possess a common regulatory element upstream of their start sites. Such DNA regulatory sequences are called response elements; they are short sequences that typically contain consensus sequences (Table 17.1) at varying distances from the gene being regulated. The response elements are binding sites for transcriptional activators. A transcriptional activator protein binds to the response element and elevates transcription. The same response element may be present in different genes, allowing multiple genes to be activated by the same stimulus.
| Response Element | Responds to | Consensus Sequence |
|---|---|---|
| Heat-shock element | Heat and other stress | CNNGAANNTCCNNG |
| Glucocorticoid response element | Glucocorticoids | TGGTACAAATGTTCT |
| Phorbol ester response element | Phorbal esters | TGACTCA |
| Serum response element | Serum | CCATATTAGG |
| Source: After B. Lewin, Genes IV (Oxford University press, 1994), p. 880. | ||
A single eukaryotic gene may be regulated by several different response elements. For example, the metallothionein gene protects cells from the toxicity of heavy metals by encoding a protein that binds to heavy metals and removes them from cells. The basal transcription apparatus assembles around the TATA box, just upstream of the transcription start site for the metallothionein gene, but the apparatus alone is capable of only low rates of transcription.
Other response elements found upstream of the metallothionein gene contribute to increasing its rate of transcription. For example, several copies of a metal response element (MRE) are upstream of the metallothionein gene (Figure 17.9). Heavy metals stimulate the binding of activator proteins to MREs, which elevates the rate of transcription of the metallothionein gene. Because there are multiple copies of the MRE, high rates of transcription are induced by metals. Two enhancers also are located in the upstream region of the metallothionein gene; one enhancer contains a response element known as TRE, which stimulates transcription in the presence of an activated protein called AP1. A third response element called GRE is located approximately 250 nucleotides upstream of the metallothionein gene and stimulates transcription in response to certain hormones.
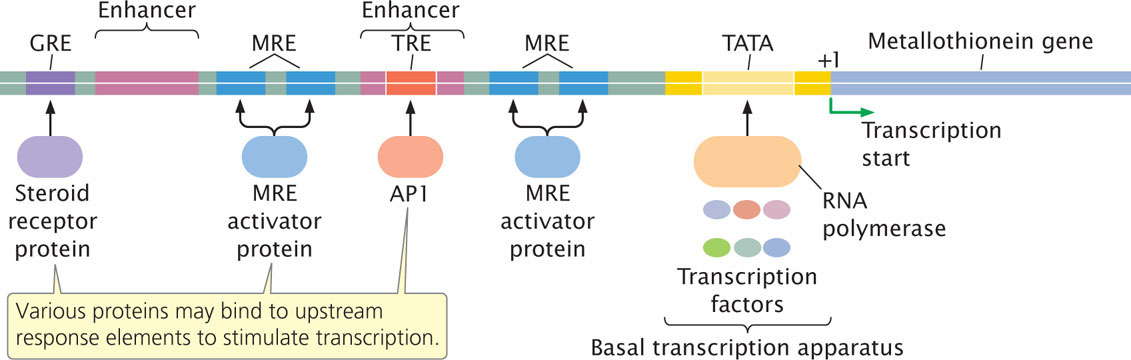
483
This example illustrates a common feature of eukaryotic transcriptional control: a single gene may be activated by several different response elements found in both promoters and enhancers. Multiple response elements allow the same gene to be activated by different stimuli. At the same time, the presence of the same response element in different genes allows a single stimulus to activate multiple genes. In this way, response elements allow complex biochemical responses in eukaryotic cells.