18.3 Mutations Are the Focus of Intense Study by Geneticists
Because mutations often have detrimental effects, they are frequently studied by geneticists. These studies have included the development of tests to determine the mutagenic properties of chemical compounds and the investigation of human populations tragically exposed to high levels of radiation.
Detecting Mutations with the Ames Test
People in industrial societies are surrounded by a multitude of artificially produced chemicals: more than 50,000 different chemicals are in commercial and industrial use today, and from 500 to 1000 new chemicals are introduced each year. Some of these chemicals are potential carcinogens, and many natural products are also potentially carcinogenic. One method for testing the cancer-causing potential of substances is to administer them to laboratory animals (rats or mice) and compare the incidence of cancer in the treated animals with that of control animals. Unfortunately, these tests are time-consuming and expensive. Furthermore, the ability of a substance to cause cancer in rodents is not always indicative of its effect on humans. After all, we aren’t rats!
In 1974, Bruce Ames developed a simple test for evaluating the potential of chemicals to cause cancer. The Ames test is based on the principle that both cancer and mutations result from damage to DNA, and the results of experiments have demonstrated that 90% of known carcinogens are also mutagens. Ames proposed that mutagenesis in bacteria could serve as an indicator of carcinogenesis in humans.
The Ames test uses different auxotrophic strains of the bacterium Salmonella typhimurium that have defects in the lipopolysaccharide coat, which normally protects the bacteria from chemicals in the environment. Furthermore, the DNA-repair system in these strains has been inactivated, enhancing their susceptibility to mutagens.
The most-recent version of the test (called Ames II) uses several auxotrophic strains that detect different types of base-pair substitutions. Other strains detect different types of frameshift mutations. Each strain carries a his− mutation, which renders it unable to synthesize the amino acid histidine, and the bacteria are plated onto medium that lacks histidine (Figure 18.22). Only bacteria that have undergone a reverse mutation of the histidine gene (his− → his+) are able to synthesize histidine and grow on the medium, which makes these mutations easy to detect. Different dilutions of a chemical to be tested are added to plates inoculated with the bacteria, and the number of mutated bacterial colonies that appear on each plate is compared with the number that appear on control plates with no chemical (i.e., that arose through spontaneous mutation). Any chemical that significantly increases the number of colonies appearing on a treated plate is mutagenic and probably also carcinogenic.
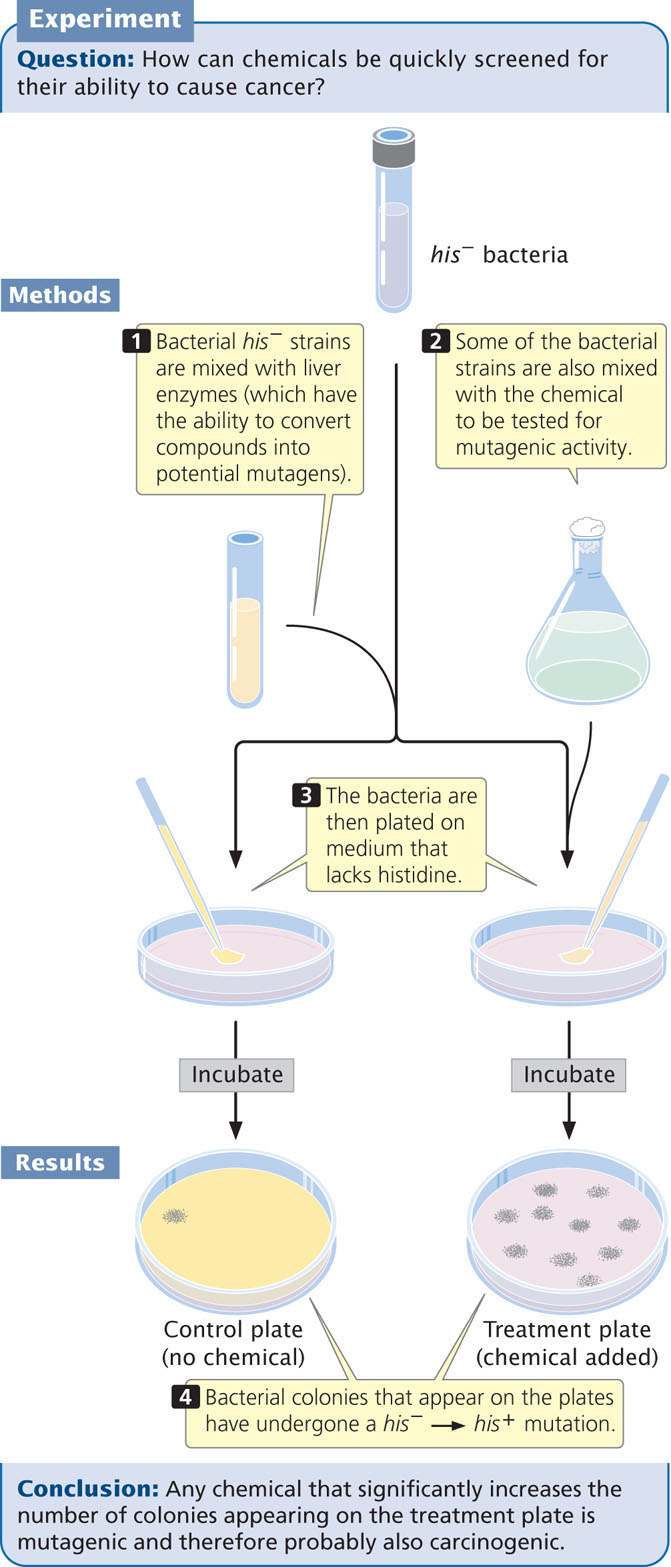
Some compounds are not active carcinogens but can be converted into cancer-causing compounds in the body. To make the Ames test sensitive for such potential carcinogens, a compound to be tested is first incubated in mammalian liver extract that contains metabolic enzymes.
The Ames test has been applied to thousands of chemicals and commercial products. An early demonstration of its usefulness was the discovery, in 1975, that many hair dyes sold in the United States contained compounds that were mutagenic to bacteria. These compounds were then removed from most hair dyes.
CONCEPTS
The Ames test uses his− strains of bacteria to test chemicals for their ability to produce his− → his+ mutations. Because mutagenic activity and carcinogenic potential are closely correlated, the Ames test is widely used to screen chemicals for their cancer-causing potential.
510
Radiation Exposure in Humans
People are routinely exposed to low levels of radiation from cosmic, medical, and environmental sources, but there have also been tragic events that produced exposures of much higher degree.
On August 6, 1945, a high-flying American airplane dropped a single atomic bomb on the city of Hiroshima, Japan. The explosion devastated an area of the city measuring 4.5 square miles, killed from 90,000 to 140,000 people, and injured almost as many (Figure 18.23). Three days later, the United States dropped an atomic bomb on the city of Nagasaki, this time destroying an area measuring 1.5 square miles and killing between 60,000 and 80,000 people. Huge amounts of radiation were released during these explosions, and many people were exposed.

After the war, a joint Japanese–U.S. effort was made to study the biological effects of radiation exposure on the survivors of the atomic blasts and their children. Somatic mutations were examined by studying radiation sickness and cancer among the survivors; germ-line mutations were assessed by looking at birth defects, chromosome abnormalities, and gene mutations in children born to people that had been exposed to radiation.
Geneticist James Neel and his colleagues examined almost 19,000 children of people who were within 2000 meters (1.2 miles) of the center of the atomic blast at Hiroshima or Nagasaki, along with a similar number of children whose parents did not receive radiation exposure. Radiation doses were estimated for a child’s parents on the basis of careful assessment of the parents’ location, posture, and position at the time of the blast. A blood sample was collected from each child, and gel electrophoresis was used to investigate amino acid substitutions in 28 proteins. When rare variants were detected, blood samples from the child’s parents also were analyzed to establish whether the variant was inherited or a new mutation.
511
Of a total of 289,868 genes examined by Neel and his colleagues, only one mutation was found in the children of exposed parents; no mutations were found in the control group. From these findings, a mutation rate of 3.4 × 10–6 was estimated for the children whose parents were exposed to the blast, which is within the range of spontaneous mutation rates observed for other eukaryotes. Neel and his colleagues also examined the frequency of chromosome mutations, sex ratios of children born to exposed parents, and frequencies of chromosome aneuploidy. There was no evidence in any of these assays for increased mutations among the children of the people who were exposed to radiation from the atomic explosions, suggesting that germ-line mutations were not elevated.
Animal studies clearly show that radiation causes germline mutations; so why was there no apparent increase in germ-line mutations among the inhabitants of Hiroshima and Nagasaki? The exposed parents did exhibit an increased incidence of leukemia and other types of cancers; so somatic mutations were clearly induced. The answer to the question is not known, but the lack of germ-line mutations may be due to the fact that those persons who received the largest radiation doses died soon after the blasts. Additional insight into the genetic effects of radiation have come from studies of people exposed to radiation in the Chernobyl nuclear accident in 1986 and other nuclear accidents, as well as exposure to radiation used in medicine and industry.