21.2 Several Molecular Processes Lead to Epigenetic Changes
Epigenetics alters the expression of genes; these alterations are stable enough to be transmitted through mitosis (and sometimes meiosis) but can also be changed. Most evidence suggests that epigenetic effects are brought about by physical changes to chromatin structure. In Chapter 11 we considered a number of chemical changes in DNA and histone proteins that affect chromatin structure, including DNA methylation, modification of histone proteins, and repositioning of nucleosomes. In Chapter 17, we discussed the role that these alterations have on the expression of genes. These chromatin changes are thought to play a role in epigenetic traits. Chapter 14 discussed small RNA molecules, some of which play an important role in bringing about epigenetic changes.
We will consider three types of molecular mechanisms that alter chromatin structure and underlie many epigenetic phenotypes: (1) changes in patterns of DNA methylation; (2) chemical modifications of histone proteins; and (3) RNA molecules that affect chromatin structure and gene expression.
CONCEPTS
Many epigenetic phenotypes are the result of alterations to chromatin structure, mediated through three major processes: DNA methylation, histone modification, and RNA molecules.
DNA Methylation
The best understood mechanism of epigenetic change is methylation of DNA. DNA methylation refers to the addition of methyl groups to the nucleotide bases. In eukaryotes, the predominant type of DNA methylation is the methylation of cytosine to produce 5-methylcytosine (Figure 21.2a). As discussed in Chapter 17, DNA methylation is often associated with repression of transcription.
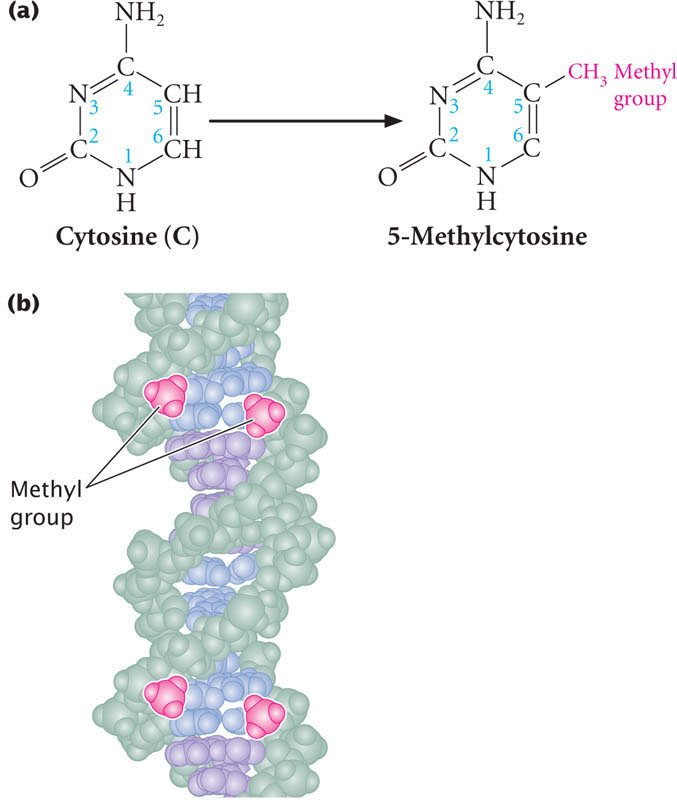
DNA methylation often occurs on cytosine bases that are immediately adjacent to guanine nucleotides, referred to as CpG dinucleotides (where p represents the phosphate group that connects C and G nucleotides). In CpG dinucleotides, cytosine nucleotides on the two DNA strands are diagonally across from one another. Usually both cytosine bases are methylated, so that methyl groups occur on both DNA strands, as shown below and in Figure 21.2b.
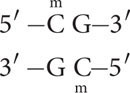
In plants, DNA methylation also occurs at CpNpG trinucleotides, where N represents a nucleotide with any base.
616
Some DNA regions have many CpG dinucleotides and are referred to as CpG islands. In mammalian cells, CpG islands are often located in or near the promoters of genes. These CpG islands are usually not methylated when genes are being actively transcribed. However, methylation of CpG islands near a gene leads to repression of transcription.
Cells repress and activate genes by methylating and demethylating cytosine bases. Enzymes called DNA methyltransferases methylate DNA by adding methyl groups to cytosine bases to create 5-methylcytosine. Other enzymes called demethylases remove methyl groups, converting 5-methylcytosine back to cytosine (see Chapter 17).
Maintenance of Methylation
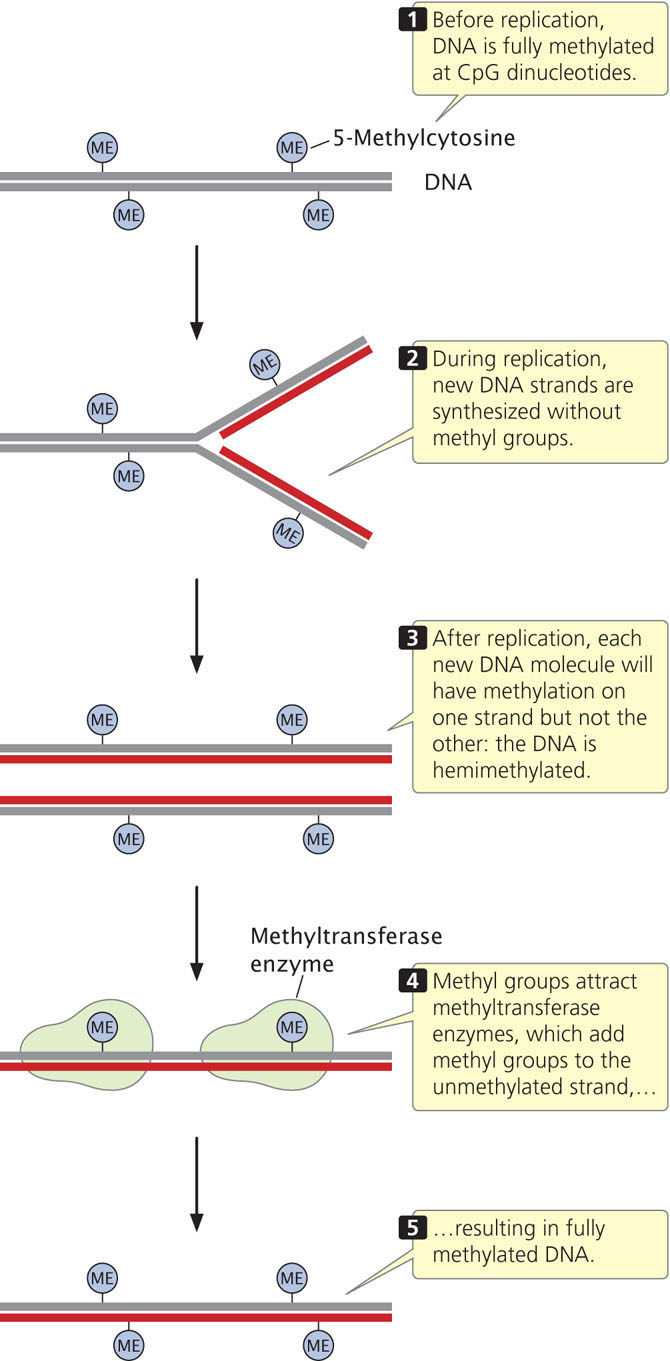
The fact that epigenetic changes are passed to other cells and (sometimes) to future generations means that changes in chromatin structure associated with epigenetic phenotypes must be faithfully maintained when chromosomes replicate. How are epigenetic changes retained and replicated through the process of cell division?
Methylation of CpG sequences means that two methylated cytosine bases sit diagonally across from each other on opposing strands. Before replication, cytosine bases on both strands are methylated (Figure 21.3). Immediately after semiconservative replication, the cytosine base on the template strand will be methylated, but the cytosine base on the newly replicated strand will be unmethylated. Special methyltransferase enzymes recognize the hemimethylated state of CpG dinucleotides and add methyl groups to the unmethylated cytosine bases, creating two new DNA molecules that are fully methylated. In this way, the methylation pattern of DNA is maintained across cell division.  TRY PROBLEM 26
TRY PROBLEM 26
617
DNA Methylation in Honeybees
A remarkable example of epigenetics is seen in honeybees. Queen bees and worker bees are both female, but the resemblance ends there. The queen is large and develops functional ovaries, whereas workers are small and sterile (Figure 21.4). The queen goes on a mating flight and spends her entire life reproducing, whereas workers spend all of their time collecting nectar and pollen, tending the queen, and raising her offspring. In spite of these significant differences in anatomy, physiology, and behavior, queens and workers are genetically the same; both develop from ordinary eggs. How they differ is in diet: worker bees produce and feed a few female larvae a special substance called royal jelly, which causes these larvae to develop as queens. Other larvae are fed ordinary bee food, and they develop as workers. This simple difference in diet greatly affects gene expression, causing different genes to be activated in queens and workers and resulting in a very different set of phenotypic traits.

How royal jelly affects gene expression has long been a mystery, but research now suggests that it changes an epigenetic mark. In 2008 Ryszard Kucharski and his colleagues demonstrated that royal jelly silences the expression of a key gene called Dnmt3, whose product normally adds methyl groups to DNA. With Dnmt3 shut down, bee DNA is less methylated and many genes that are normally silenced in workers are expressed, leading to the development of queen characteristics. Kucharski and his coworkers demonstrated the importance of DNA methylation in queen development by injecting into bee larvae small RNA molecules (small interfering RNAs, or siRNAs; see Chapter 14) that specifically inhibited the expression of Dnmt3. These larvae had lower levels of DNA methylation and many developed as queens with fully functional ovaries. This experiment demonstrated that royal jelly brings about epigenetic changes (less DNA methylation), which are transmitted through cell division and modify developmental pathways, eventually leading to a queen bee.  TRY PROBLEM 25
TRY PROBLEM 25
Repression of Transcription by DNA Methylation
How does DNA methylation suppress gene expression? The methyl group of 5-methylcytosine sits within the major groove of the DNA, which is recognized by many DNA binding proteins. The presence of the methyl group in the major groove inhibits the binding of transcription factors and other proteins required for transcription to occur. 5-methylcytosine also attracts certain proteins that directly repress transcription. In addition, DNA methylation attracts histone deacetylase enzymes, which remove acetyl groups from the tails of histone proteins, altering chromatin structure in a way that represses transcription (see Chapter 17).
CONCEPTS
Cytosine bases are often methylated to form 5-methylcytosine, which is associated with repression of transcription. DNA methylation is stably maintained through replication by methyltransferase enzymes that recognize the hemimethylated state of CpG dinucleotides and add methyl groups to the unmethylated cytosine bases.
 CONCEPT CHECK 1
CONCEPT CHECK 1
Which of the following is true of CpG islands?
- They are methylated near promoters of actively transcribed genes.
- They are unmethylated near promoters of actively transcribed genes.
- Acetylation of CpG islands leads to repression of transcription.
- CpG islands code for RNA molecules that activate transcription.
Histone Modifications
Epigenetic changes can also occur through modification of histone proteins. In eukaryotic cells, DNA is complexed to histone proteins in the form of nucleosomes, which are the basic repeating units of chromatin structure (see Chapter 11). More than 100 different posttranslational modifications of histone proteins have been detected. Many of these modifications take place in the positively charged tails of the histone proteins, which interact with the DNA and affect chromatin structure. Modifications to histones include the addition of phosphates, methyl groups, acetyl groups, and ubiquitin to their tails. These modifications often alter chromatin structure and affect transcription of genes (see Chapter 17). The modifications may also serve as binding sites for proteins such as transcription factors that are required for transcription.
618
The addition of acetyl groups to amino acids in the histone tails (histone acetylation) generally destabilizes chromatin structure, causing it to assume a more open configuration and is associated with increased transcription (see Figure 17.2 in Chapter 17). The addition of methyl groups to histones (histone methylation) also alters chromatin structure, but the effect varies depending on the specific amino acid that is methylated; some types of histone methylation are associated with increased transcription and other types are associated with decreased transcription. For example, the addition of three methyl groups to lysine 4 in the H3 histone (H3K4me3, K stands for lysine) is often found near transcriptionally active genes. Methylation of lysine 36 in the H3 histone (H3K36me3) is also associated with increased transcription. On the other hand, the addition of three methyl groups to lysine 9 in H3 (H3K9me3) and to lysine 20 in histone 4 (H4K20me3) is associated with repression of transcription. Many additional histone marks have also been shown to associate with the level of transcription. These types of modifications have been called epigenetic marks.
Histone modifications are added and removed by special proteins. The polycomb group (PcG) proteins are a large group of proteins that repress transcription by modifying histones. These modifications alter chromatin structure so that the DNA is not accessible to transcription factors, RNA polymerase, and other proteins required for transcription. For example, polycomb repressive complex 2 (PRC2) adds two or three methyl groups to lysine 27 of histone H3, creating the H3K27me3 epigenetic mark that represses transcription.
Many of the enzymes and proteins that produce epigenetic marks cannot bind to specific DNA sequences by themselves. Thus, they must be recruited to specific targets on the chromosome. Sequence-specific transcription factors, pre-existing chromatin marks, and noncoding RNA molecules serve to recruit histone-modifying enzymes to specific sites.
Research indicates that single histone modifications, such as those mentioned here, do not individually determine the transcriptional activity of a gene. Rather, it is the combined presence of multiple epigenetic marks that determine the activity level. There is also considerable “crosstalk” between epigenetic marks—one histone mark may affect whether additional marks occur nearby and how they function. Crosstalk occurs because histone modifications attract enzymes and proteins that modify other histones. Histone modifications not only affect transcription, but can also influence other molecular processes such as DNA repair and cell cycle checkpoint signaling. For example, ubiquitination of histone H2B is required for repair of double-strand breaks in DNA. This modification leads to other histone modifications, such as methylation of lysine 79 of H3 (H3K79me); these modifications alter chromatin structure and allow access of proteins that repair double-strand breaks.
Maintenance of Histone Modifications
The process by which histone modifications are maintained across cell division is not as well understood as that of DNA methylation. There is no universal mechanism for maintaining histone modifications; different types of modifications are undoubtedly maintained by different mechanisms.
Several models have been proposed to explain how histone modifications are faithfully transmitted to daughter cells. During the process of DNA replication, nucleosomes are disrupted and the original histone proteins are distributed randomly between the two new DNA molecules. Newly synthesized histones are then added to complete the formation of new nucleosomes (see Chapter 12). Most models assume that after replication the epigenetic marks remain on the original histones, and these marks recruit enzymes that make similar changes to the new histones. For example, PRC2 adds the H3K27me3 epigenetic mark to histones. PRC2 preferentially targets histones in chromatin that already contains an H327me3 mark, ensuring that any new nucleosomes that are added after replication will also become methylated. In this way the histone modifications can be maintained across cell division.
CONCEPTS
Modification of histone proteins, including the addition of methyl groups, acetyl groups, phosphates, and ubiquitination alter chromatin structure. Some of these modifications are passed to daughter cells during cell division and to future generations.
Epigenetic Effects by RNA Molecules
Evidence increasingly demonstrates that RNA molecules play an important role in bringing about epigenetic effects. The first discovered and still best understood example of RNA mediating epigenetic change is X inactivation, in which a long, noncoding RNA called Xist suppresses transcription on one of the X chromosomes in female mammals. Another example involves paramutation in corn, in which an epigenetically altered allele induces a change in another allele that then gets transmitted to future generations. Paramutation in corn is brought about by siRNAs (see Chapter 14). Both of these examples will be discussed later in this chapter.
Different mechanisms are involved in epigenetic changes through RNA molecules. In the case of X inactivation, the Xist RNA coats one X chromosome and then attracts PRC2, which deposits methyl groups on lysine 27 of histone H3, creating the H3K27me3 epigenetic mark that alters chromatin structure and represses transcription.
Other examples of RNA-associated epigenetic phenotypes occur through siRNA molecules that silence genes and transposable elements (see Chapter 18) by directing DNA methylation or histone modifications to specific DNA sequences. In addition, research has demonstrated that epigenetic processes such as methylation and histone modification influence the expression of microRNAs (see Chapter 14) which, in turn, play an important role in regulating other genes. MicroRNAs also control the expression of genes that produce epigenetic effects, such as enzymes that methylate DNA and modify histone proteins. How RNA-based epigenetic changes are maintained across cell divisions is less clear, although some apparently involve small RNAs that are transmitted through the cytoplasm.
619
CONCEPTS
RNA molecules bring about modification of chromatin by a variety of processes.
 CONCEPT CHECK 2
CONCEPT CHECK 2
Which is not a major mechanism of epigenetic change?
- DNA methylation.
- Alteration of a DNA base sequence in a promoter.
- Histone acetylation.
- Action of RNA molecules.