22.2 Pattern Formation in Drosophila Serves As a Model for the Genetic Control of Development
Pattern formation consists of the developmental processes that lead to the shape and structure of complex, multicellular organisms. One of the best-studied systems for the genetic control of pattern formation is the early embryonic development of Drosophila melanogaster. Geneticists have isolated a large number of mutations in fruit flies that influence all aspects of their development, and molecular analysis of these mutations has provided much information about how genes control early development in Drosophila.
The Development of the Fruit Fly
An adult fruit fly possesses three basic body parts: head, thorax, and abdomen (Figure 22.4). The thorax consists of three segments: the first thoracic segment carries a pair of legs; the second thoracic segment carries a pair of legs and a pair of wings; and the third thoracic segment carries a pair of legs and the halteres (rudiments of the second pair of wings found in most other insects). The abdomen consists of a number of segments.
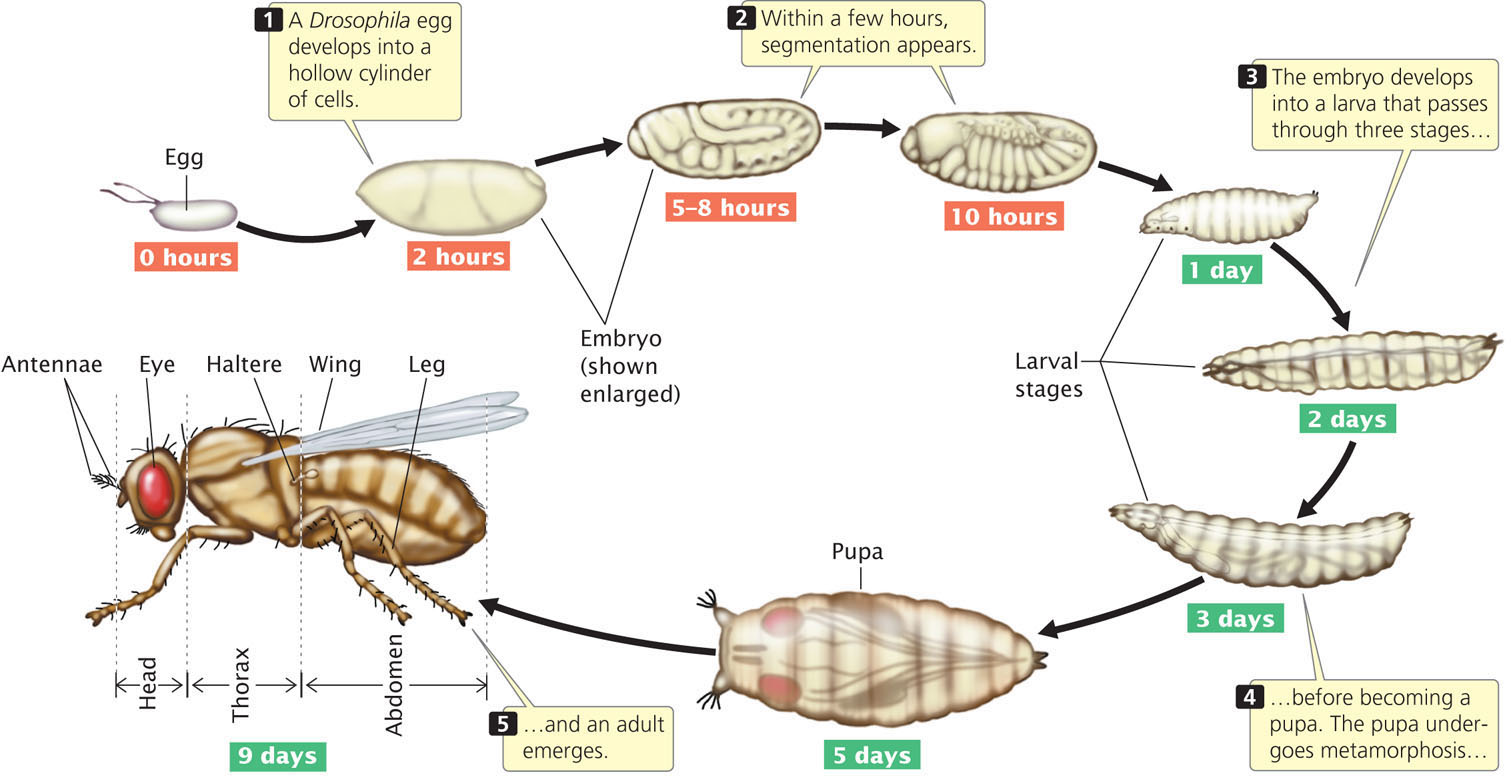
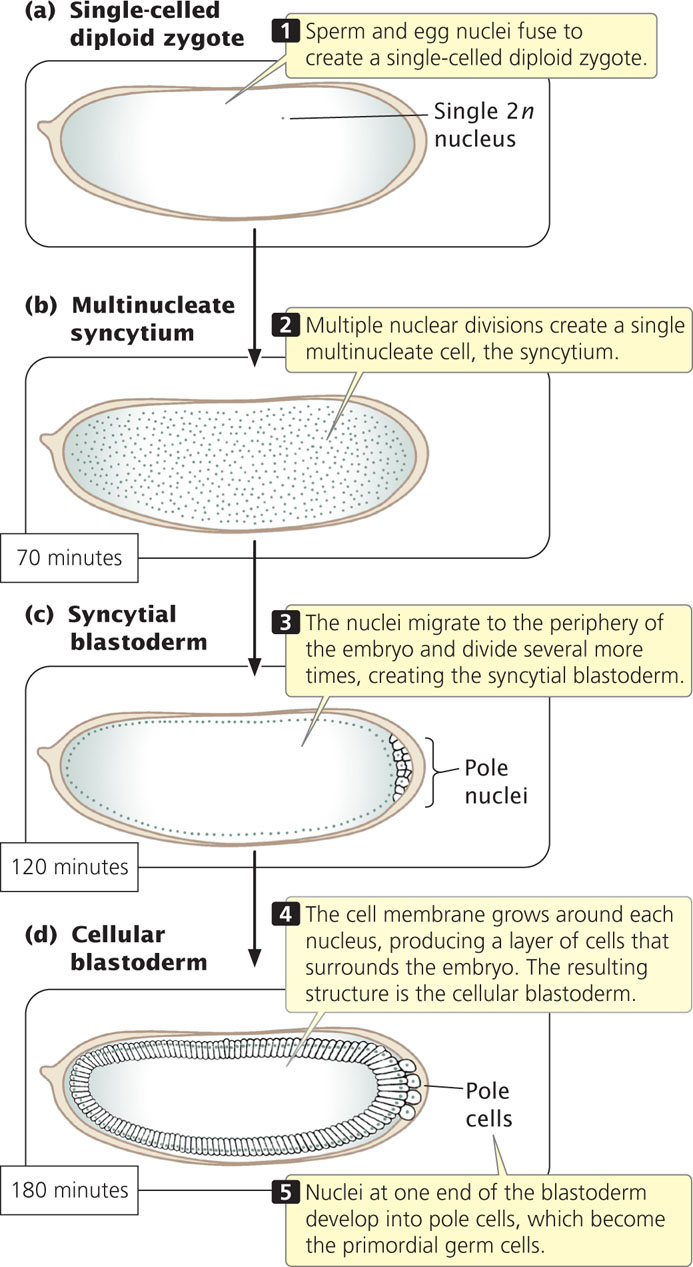
When a Drosophila egg has been fertilized, its diploid nucleus (Figure 22.5a) immediately divides nine times without division of the cytoplasm, creating a single, multinucleate cell (Figure 22.5b). These nuclei are scattered throughout the cytoplasm but later migrate toward the periphery of the embryo and divide several more times (Figure 22.5c). Next, the cell membrane grows inward and around each nucleus, creating a layer of approximately 6000 cells at the outer surface of the embryo (Figure 22.5d). Nuclei at one end of the embryo develop into pole cells, which eventually give rise to germ cells. The early embryo then undergoes further development in three distinct stages: (1) the anterior–posterior axis and the dorsal–ventral axis of the embryo are established (Figure 22.6a); (2) the number and orientation of the body segments are determined (Figure 22.6b); and (3) the identity of each individual segment is established (Figure 22.6c). Different sets of genes control each of these three stages (Table 22.1).

| Developmental Stage | Genes |
|---|---|
| Establishment of main body axes | Egg-polarity genes |
| Determination of number and polarity of body segments | Segmentation genes |
| Establishment of identity of each segment | Homeotic genes |
637
Egg-Polarity Genes
The egg-polarity genes (genes that determine polarity, or direction) play a crucial role in establishing the two main axes of development in fruit flies. You can think of these axes as the longitude and latitude of development: any location in the Drosophila embryo can be defined in relation to these two axes.
The egg-polarity genes are transcribed into mRNAs in the course of egg formation in the maternal parent, and these mRNAs become incorporated into the cytoplasm of the egg. After fertilization, the mRNAs are translated into proteins that play an important role in determining the anterior–posterior and dorsal–ventral axes of the embryo. Because the mRNAs of the polarity genes are produced by the female parent and influence the phenotype of the offspring, the traits encoded by them are examples of genetic maternal effects (see Chapter 5).
638
There are two sets of egg-polarity genes: one set determines the anterior–posterior axis, and the other determines the dorsal–ventral axis. These genes work by setting up concentration gradients of morphogens within the developing embryo. A morphogen is a protein that varies in concentration and elicits different developmental responses at different concentrations. Egg-polarity genes function by producing proteins that become asymmetrically distributed in the cytoplasm, giving the egg polarity. This asymmetrical distribution may take place in a couple of ways. An mRNA may be localized to particular regions of the egg cell, leading to an abundance of the protein in those regions when the mRNA is translated. Alternatively, the mRNA may be randomly distributed, but the protein that it encodes may become asymmetrically distributed by a transport system that delivers it to particular regions of the cell, by regulation of its translation, or by its removal from particular regions by selective degradation.
Determination of the Dorsal–Ventral Axis
The dorsal–ventral axis defines the back (dorsum) and belly (ventrum) of a fly (see Figure 22.6). At least 12 different genes determine this axis, one of the most important being a gene called dorsal. The dorsal gene is transcribed and translated in the maternal ovary, and the resulting mRNA and protein are transferred to the egg during oogenesis. In a newly laid egg, mRNA and protein encoded by the dorsal gene are uniformly distributed throughout the cytoplasm but, after the nuclei have migrated to the periphery of the embryo (see Figure 22.5c), Dorsal protein becomes redistributed. Along one side of the embryo, Dorsal protein remains in the cytoplasm; this side will become the dorsal surface. Along the other side, Dorsal protein is taken up into the nuclei; this side will become the ventral surface. At this point, there is a smooth gradient of increasing nuclear Dorsal concentration from the dorsal to the ventral side (Figure 22.7).

The nuclear uptake of Dorsal protein is thought to be governed by a protein called Cactus, which binds to Dorsal protein and traps it in the cytoplasm. The presence of yet another protein, called Toll, leads to the phosphorylation of Cactus, causing it to be degraded. When Cactus is degraded, Dorsal is released and can move into the nucleus. Together, Cactus and Toll regulate the nuclear distribution of Dorsal protein, which in turn determines the dorsal–ventral axis of the embryo.
Inside the nucleus, Dorsal protein acts as a transcription factor, binding to regulatory sites on the DNA and activating or repressing the expression of other genes (Table 22.2). High nuclear concentration of Dorsal protein (as in cells on the ventral side of the embryo) activates a gene called twist, which causes ventral tissues to develop. Low nuclear concentrations of Dorsal protein (as in cells on the dorsal side of the embryo), activate a gene called decapentaplegic, which specifies dorsal structures. In this way, the ventral and dorsal sides of the embryo are determined.
| Gene | Where Expressed | Action of Gene Product |
|---|---|---|
| dorsal | Ovary | Affects the expression of genes such as twist and decapentaplegic |
| cactus | Ovary | Traps Dorsal protein in the cytoplasm |
| toll | Ovary | Leads to the phosphorylation of Cactus, which is then degraded, releasing Dorsal to move into the nuclei of ventral cells |
| twist | Embryo | Takes part in the development of mesodermal tissues* |
| decapentaplegic | Embryo | Takes part in the development of gut structures |
| *One of the three primary tissue layers in the early embryo. | ||
639
Determination of the Anterior–Posterior Axis
One of the most important early developmental events is the determination of the anterior (head) and posterior (butt) ends of an animal. We will consider several key genes that establish this anterior–posterior axis of the Drosophila embryo (Table 22.3). An important gene in this regard is bicoid, which is first transcribed in the ovary of an adult female during oogenesis. The bicoid mRNA becomes incorporated into the cytoplasm of the egg; as it passes into the egg, bicoid mRNA becomes anchored to the anterior end of the egg by part of its 3′ end. This anchoring causes bicoid mRNA to become concentrated at the anterior end (Figure 22.8a). (A number of other genes that are active in the ovary are required for proper localization of bicoid mRNA in the egg.) When the egg has been laid, bicoid mRNA is translated into Bicoid protein. Because most of the mRNA is at the anterior end of the egg, Bicoid protein is synthesized there and forms a concentration gradient along the anterior–posterior axis of the embryo, with a high concentration at the anterior end and a low concentration at the posterior end. This gradient is maintained by the continuous synthesis of Bicoid protein and its short half-life.
| Gene | Where Expressed | Action |
|---|---|---|
| bicoid | Ovary | Regulates expression of genes responsible for anterior structures; stimulates hunchback |
| nanos | Ovary | Regulates expression of genes responsible for posterior structures; inhibits translation of hunchback mRNA |
| hunchback | Embryo | Regulates transcription of genes responsible for anterior structures |
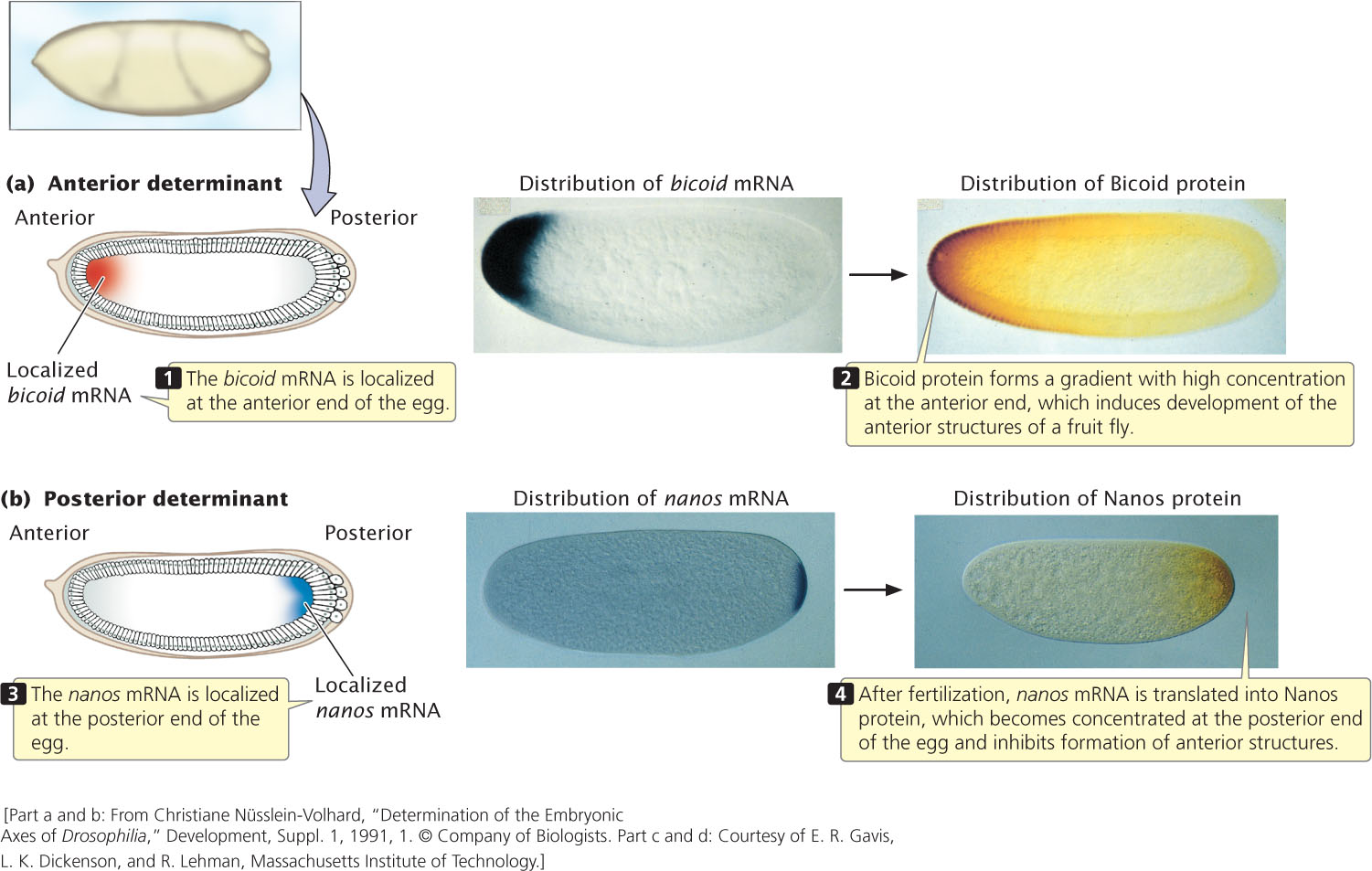
The high concentration of Bicoid protein at the anterior end induces the development of anterior structures such as the head of the fruit fly. It stimulates the development of anterior structures by binding to regulatory sequences in the DNA and influencing the expression of other genes. One of the most important of the genes stimulated by Bicoid protein is hunchback, which is required for the development of the head and thoracic structures of the fruit fly.
The development of the anterior–posterior axis is also greatly influenced by a gene called nanos, an egg-polarity gene that acts at the posterior end of the axis. The nanos gene is transcribed in the adult female, and the resulting mRNA becomes localized at the posterior end of the egg (Figure 22.8b). After fertilization, nanos mRNA is translated into Nanos protein, which diffuses slowly toward the anterior end. The Nanos protein gradient is opposite that of the Bicoid protein: Nanos is most concentrated at the posterior end of the embryo and is least concentrated at the anterior end. Nanos protein inhibits the formation of anterior structures by repressing the translation of hunchback mRNA. The synthesis of the Hunchback protein is therefore stimulated at the anterior end of the embryo by Bicoid protein and is repressed at the posterior end by Nanos protein. This combined stimulation and repression results in a Hunchback protein concentration gradient along the anterior–posterior axis that, in turn, affects the expression of other genes and helps determine the anterior and posterior structures.
640
CONCEPTS
The major axes of development in early fruit-fly embryos are established as a result of initial differences in the distribution of specific mRNAs and proteins encoded by genes in the female parent (genetic maternal effect). These differences in distribution establish concentration gradients of morphogens, which cause different genes to be activated in different parts of the embryo.
 CONCEPT CHECK 2
CONCEPT CHECK 2
High concentration of which protein stimulates the development of anterior structures?
- Dorsal.
- Toll.
- Bicoid.
- Nanos.
Segmentation Genes
Like all insects, the fruit fly has a segmented body plan. When the basic dorsal–ventral and anterior–posterior axes of the fruit-fly embryo have been established, segmentation genes control the differentiation of the embryo into individual segments. These genes affect the number and organization of the segments, and mutations in them usually disrupt whole sets of segments. The approximately 25 segmentation genes in Drosophila are transcribed after fertilization, so they don’t exhibit a genetic maternal effect and their expression is regulated by the Bicoid and Nanos protein gradients.
The segmentation genes fall into three classes as shown in Table 22.4 and Figure 22.9. The three classes act sequentially, affecting progressively smaller regions of the embryo. First, the products of the egg-polarity genes activate or repress gap genes, which divide the embryo into broad regions. The gap genes, in turn, regulate pair-rule genes, which affect the development of pairs of segments. Finally, the pair-rule genes influence segment-polarity genes, which guide the development of individual segments.
| Class of Gene | Effect of Mutations | Examples of Genes |
|---|---|---|
| Gap genes | Delete adjacent segments | hunchback, Krüppel, knirps, giant, tailless |
| Pair-rule genes | Delete same part of pattern in every other segment | runt, hairy, fushi tarazu, even-skipped, odd-paired, sloppy paired, odd-skipped |
| Segment-polarity genes | Affect polarity of segment; part of segment replaced by mirror image of part of another segment | engrailed, wingless, gooseberry, cubitus interruptus, patched, hedgehog, disheveled, costal-2, fused |
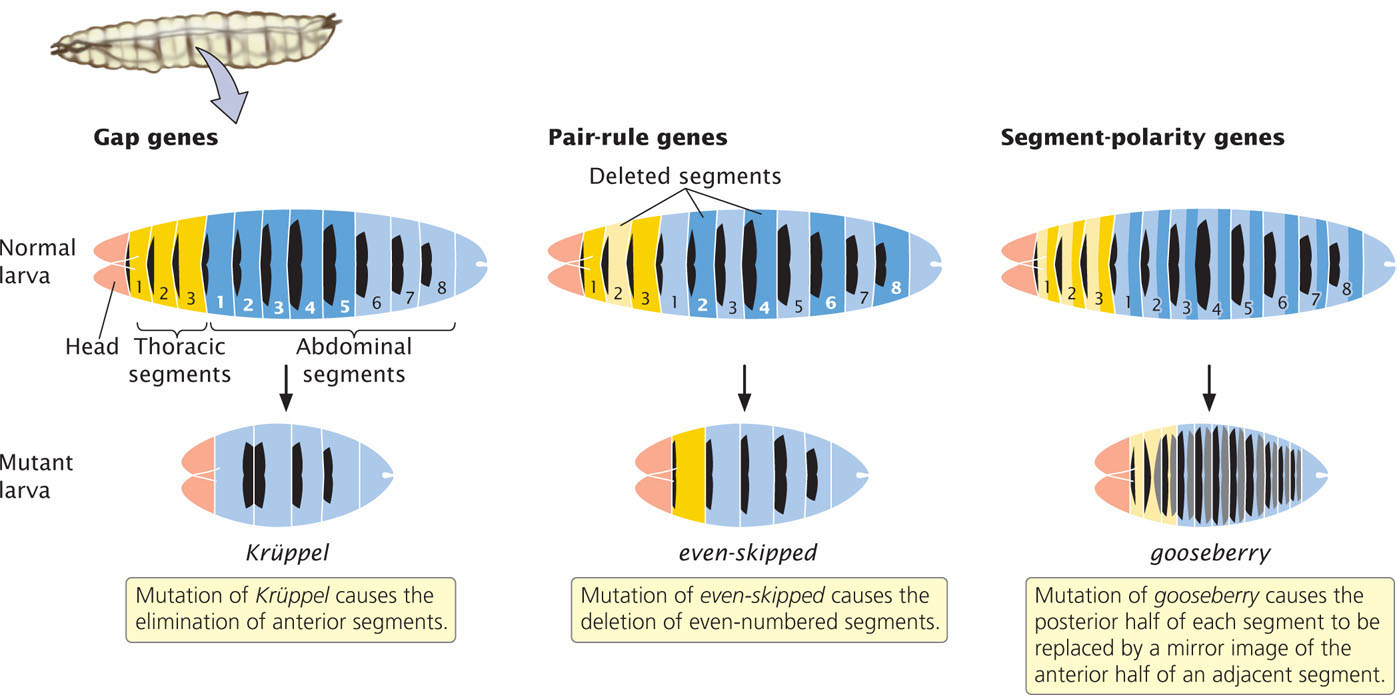
Gap genes define large sections of the embryo; mutations in these genes eliminate whole groups of adjacent segments. Mutations in the Krüppel gene, for example, cause the absence of several adjacent segments. Pair-rule genes define regional sections of the embryo and affect alternate segments. Mutations in the even-skipped gene cause the deletion of even-numbered segments, whereas mutations in the fushi tarazu gene cause the absence of odd-numbered segments. Segment-polarity genes affect the orientation of segments. Mutations in these genes cause part of each segment to be deleted and replaced by a mirror image of part or all of an adjacent segment. For example, mutations in the gooseberry gene cause the posterior half of each segment to be replaced by the anterior half of an adjacent segment.
CONCEPTS
When the major axes of the fruit-fly embryo have been established, segmentation genes determine the number, orientation, and basic organization of the body segments.
 CONCEPT CHECK 3
CONCEPT CHECK 3
The correct sequence in which the segmentation genes act is:
- segment-polarity genes → gap genes → pair-rule genes.
- gap genes → pair-rule genes → segment-polarity genes.
- segment-polarity genes → pair-rule genes → gap genes.
- gap genes → segment-polarity genes → pair-rule genes.
Homeotic Genes in Drosophila
After the segmentation genes have established the number and orientation of the segments, homeotic genes become active and determine the identity of individual segments. Eyes normally arise only on the head segment, whereas legs develop only on the thoracic segments. The products of homeotic genes activate other genes that encode these segment-specific characteristics. Mutations in the homeotic genes cause body parts to appear in the wrong segments.
641
In the late 1940s, Edward Lewis began to study homeotic mutations in Drosophila—mutations that cause bizarre rearrangements of body parts. Mutations in the Antennapedia gene, for example, cause legs to develop on the head of a fly in place of the antennae (Figure 22.10). Homeotic genes create addresses for the cells of particular segments, telling the cells where they are within the regions defined by the segmentation genes. When a homeotic gene is mutated, the address is wrong and cells in the segment develop as though they were somewhere else in the embryo.

Homeotic genes in Drosophila are expressed after fertilization and are activated by specific concentrations of the proteins produced by the gap, pair-rule, and segment-polarity genes. The homeotic gene Ultrabithorax (Ubx), for example, is activated when the concentration of Hunchback protein (a product of a gap gene) is within certain values. These concentrations exist only in the middle region of the embryo; so Ubx is expressed only in these segments.
The homeotic genes in animals encode regulatory proteins that bind to DNA; each gene contains a subset of nucleotides, called a homeobox, that are similar in all homeotic genes. The homeobox encodes 60 amino acids that serve as a DNA-binding domain; this domain is related to the helix-turn-helix motif (see Figure 16.2a). Homeoboxes are also present in segmentation genes and other genes that play a role in spatial development.
There are two major clusters of homeotic genes in Drosophila. One cluster, the Antennapedia complex, affects the development of the adult fly’s head and anterior thoracic segments. The other cluster consists of the bithorax complex and includes genes that influence the adult fly’s posterior thoracic and abdominal segments. Together, the bithorax and Antennapedia genes are termed the homeotic complex (HOM-C). In Drosophila, the bithorax complex comprises three genes, and the Antennapedia complex has five; all are located on the same chromosome (Figure 22.11). In addition to these eight genes, HOM-C contains many sequences that regulate the homeotic genes. Remarkably, the order of the genes in the HOM-C is the same as the order in which the genes are expressed along the anterior–posterior axis of the body. The genes that are expressed in the more-anterior segments are found at one end of the complex, whereas those expressed in the more-posterior end of the embryo are found at the other end of the complex (see Figure 22.11).
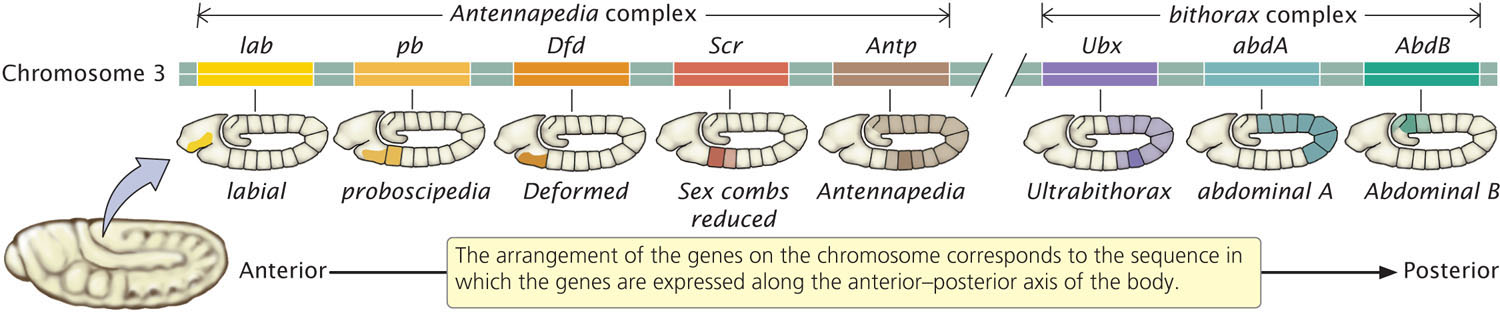
642
CONCEPTS
Homeotic genes help determine the identity of individual segments in Drosophila embryos by producing DNA-binding proteins that activate other genes. Each homeotic gene contains a consensus sequence called a homeobox, which encodes the DNA-binding domain.
 CONCEPT CHECK 4
CONCEPT CHECK 4
Mutations in homeotic genes often cause
- the deletion of segments.
- the absence of structures.
- too many segments.
- structures to appear in the wrong place.
Homeobox Genes in Other Organisms
After homeotic genes in Drosophila had been isolated and cloned, molecular geneticists set out to determine if similar genes exist in other animals; probes complementary to the homeobox of Drosophila genes were used to search for homologous genes that might play a role in the development of other animals. The search was hugely successful: homeobox-containing genes have been found in all animals, including nematodes, beetles, sea urchins, frogs, birds, and mammals. Genes with homeoboxes have even been discovered in fungi and plants, indicating that the homeobox arose early in the evolution of eukaryotes. One group of homeobox genes comprises the Hox genes, which include the homeotic complex of Drosophila described in the preceding section. Hox genes are found in all animals except sponges.
In vertebrates, there are usually four clusters of Hox genes, each of which contains from 9 to 11 genes. Mammalian Hox genes, like those in Drosophila, encode transcription factors that help determine the identity of body regions along an anterior–posterior axis. The Hox genes of other organisms often exhibit the same relation between order on the chromosome and order of their expression along the anterior–posterior axis of the embryo as that of Drosophila (Figure 22.12), but this pattern is not universal. For example, the tunicate Oikopleura dioica (a primitive relative of vertebrates) has 9 Hox genes, but the genes are scattered throughout the genome, unlike the clustered arrangement seen in most animals. Despite a lack of physical clustering, the Hox genes in O. dioica are expressed in the same anterior–posterior order as that seen in vertebrates.

The Hox genes of vertebrates also exhibit a relation between their order on the chromosome and the timing of their expression: genes at one end of the complex (those expressed at the anterior end) are expressed early in development, whereas genes at the other end (those expressed at the posterior end) are expressed later. If a Hox gene is experimentally moved to a new location within the Hox-gene complex, it is expressed in the appropriate tissue but the timing of its expression is altered, suggesting that the timing of gene expression is controlled by its physical location within the complex. Although the mechanism of this sequential control is not well understood, evidence suggests that, in mice, it is controlled by a progressive change in the methylation patterns of histone proteins, an epigenetic change that alters chromatin structure and affects transcription (see Chapter 21). Recent studies have also identified microRNA genes (see Chapter 14) within the Hox-gene clusters, and evidence suggests that miRNAs play a role in controlling the expression of some Hox genes.
Hox genes and their expression are often correlated with anatomical changes in animals, and Hox genes have been hypothesized to play an important role in the evolution of animals. For example, the lancet Branchiostoma (another primitive relative of vertebrates) has a simple body form and possesses only 14 Hox genes in a single cluster, whereas some fishes, with much more complex body architecture, have as many as 48 Hox genes in seven clusters.
643
CONNECTING CONCEPTS: The Control of Development
Development is a complex process consisting of numerous events that must take place in a highly specific sequence. Studies of fruit flies and other organisms reveal that this process is regulated by a large number of genes. In Drosophila, the dorsal–ventral axis and the anterior–posterior axis are established by maternal genes; these genes encode mRNAs and proteins that are localized to specific regions within the egg and cause specific genes to be expressed in different regions of the embryo. The proteins of these genes then stimulate other genes, which in turn stimulate yet other genes in a cascade of control. As might be expected, most of the gene products in the cascade are regulatory proteins, which bind to DNA and activate other genes.
In the course of development, successively smaller regions of the embryo are determined (Figure 22.13). In Drosophila, first, the major axes and regions of the embryo are established by egg-polarity genes. Next, patterns within each region are determined by the action of segmentation genes: the gap genes define large sections, the pair-rule genes define regional sections of the embryo and affect alternate segments, and the segment-polarity genes affect individual segments. Finally, the homeotic genes provide each segment with a unique identity. Initial gradients in proteins and mRNA stimulate localized gene expression, which produces more finely located gradients that stimulate even more localized gene expression. Developmental regulation thus becomes more and more narrowly defined.

The processes by which limbs, organs, and tissues form (called morphogenesis) are less well understood, although this pattern of generalized-to-localized gene expression is encountered frequently.  TRY PROBLEM 22
TRY PROBLEM 22
Epigenetic Changes in Development
Early development of the fruit fly is controlled in large part by the products of certain key genes selectively activating or repressing the expression of other genes. As discussed in Chapter 21, gene expression in eukaryotes is affected by epigenetic changes, and indeed epigenetics also plays an important role in development.
In Chapter 21, epigenetic changes were defined as heritable alterations to DNA and chromatin structure—alterations that affect gene expression and are passed on to other cells but are not changes to the DNA base sequence. In the course of development, a single-cell zygote divides and gives rise to many cells, which differentiate and acquire the characteristics of specific organs and tissues. Each type of cell eventually expresses a different subset of genes, producing the proteins needed for that cell type, and this program of gene expression is passed on when the differentiated cell divides.
644
The gene-expression program of cells that make up a particular organ or tissue type is often defined by epigenetic marks. As development and differentiation proceed, cells acquire epigenetic changes that turn specific sets of genes on and off. In Chapter 21, we considered several types of epigenetic marks, including DNA methylation, the modification of histone proteins, and changes due to RNA molecules. These epigenetic changes help determine which genes are expressed by a cell. In early stages of development, genes that may be required at later stages of development are often held in a transiently silent state by histone modifications. Histone modifications are generally flexible and easily reversed, and so these genes can be activated later in developmental stages. In the course of development, other genes are permanently silenced; this more-long-term silencing is often accomplished by DNA methylation.