22.6 The Development of Immunity Is Through Genetic Rearrangement
As we have seen in our consideration of animal and plant development, a basic principle of developmental biology is that every somatic cell carries an identical set of genetic information and that no genes are lost in development. Although this principle holds for most cells, there are some important exceptions, one of which concerns genes that encode immune function in vertebrates. In the development of immunity, individual segments of certain genes are rearranged into different combinations, producing immune cells that contain different genetic information and are each adapted to attack one particular foreign substance. This rearrangement and loss of genetic material is key to the power of our immune systems to protect us against almost any conceivable foreign substance.
The immune system provides protection against infection by bacteria, viruses, fungi, and parasites. The focus of an immune response is an antigen, defined as any molecule (usually a protein) that elicits an immune reaction. The immune system is remarkable in its ability to recognize an almost unlimited number of potential antigens. The body is full of proteins, so it is essential that the immune system be able to distinguish between self-antigens and foreign antigens. Occasionally, the ability to make this distinction breaks down, and the body produces an immune reaction to its own antigens, resulting in an autoimmune disease (Table 22.5).
| Disease | Tissues Attacked |
|---|---|
| Graves disease, Hashimoto thyroiditis | Thyroid gland |
| Rheumatic fever | Heart muscle |
| Systemic lupus erythematosus | Joints, skin, and other organs |
| Rheumatoid arthritis | Joints |
| Insulin-dependent diabetes mellitus | Insulin-producing cells in pancreas |
| Multiple sclerosis | Myelin sheath around nerve cells |
650
The Organization of the Immune System
The immune system contains a number of different components and uses several mechanisms to provide protection against pathogens, but most immune responses can be grouped into two major classes: humoral immunity and cellular immunity. Although it is convenient to think of these classes as separate systems, they interact and influence each other significantly.
Humoral Immunity
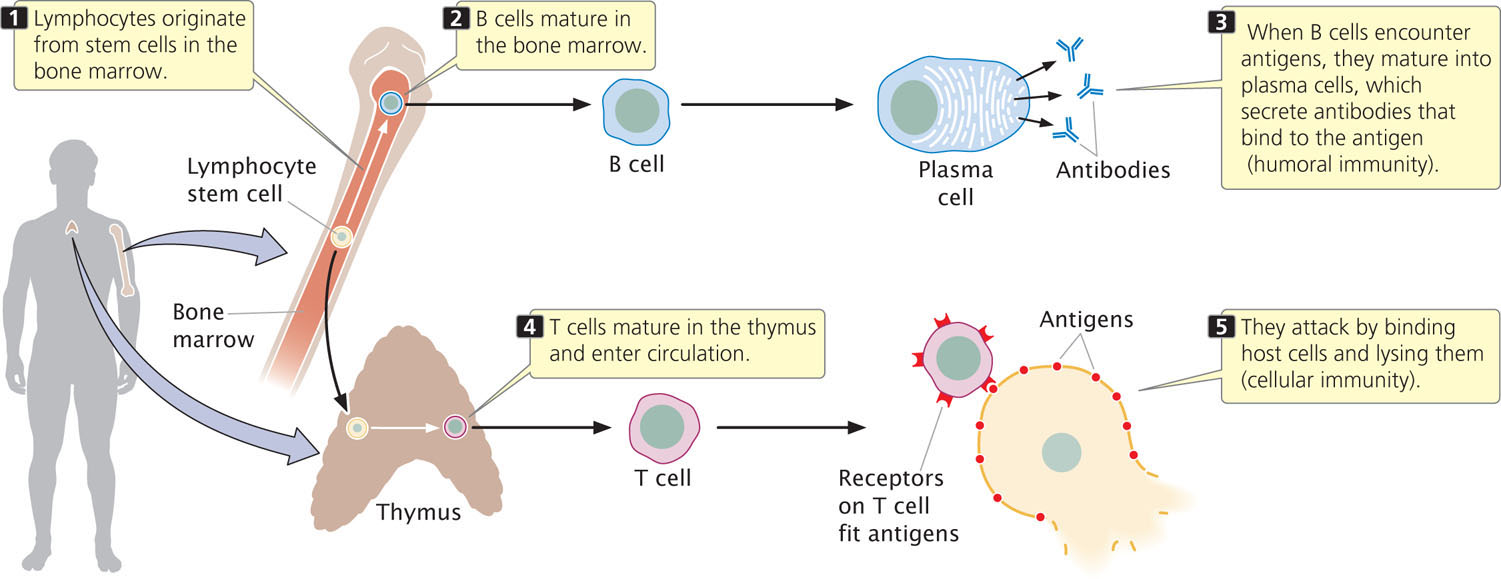
Immune function is carried out by specialized blood cells called lymphocytes, which are a type of white blood cell. Humoral immunity centers on the production of antibodies by specialized lymphocytes called B cells (Figure 22.20), which mature in the bone marrow. Antibodies are proteins that circulate in the blood and other body fluids, binding to specific antigens and marking them for destruction by phagocytic cells. Antibodies also activate a set of proteins called complement that help to lyse cells and attract macrophages.
Cellular Immunity
T cells (see Figure 22.20), are specialized lymphocytes that mature in the thymus and respond only to antigens found on the surfaces of the body’s own cells. These lymphocytes are responsible for the second type of immune response, cellular immunity.
After a pathogen such as a virus has infected a host cell, some viral antigens appear on the cell’s surface. Proteins, called T-cell receptors, on the surfaces of T cells bind to these antigens and mark the infected cell for destruction. T-cell receptors must simultaneously bind a foreign antigen and a self-antigen called a major histocompatibility complex (MHC) antigen on the cell surface (discussed later in the chapter). Not all T cells attack cells having foreign antigens; some help regulate immune responses, providing communication among different components of the immune system.
Clonal Selection
How can the immune system recognize an almost unlimited number of foreign antigens? Remarkably, each mature lymphocyte is genetically programmed to attack one and only one specific antigen: each mature B cell produces antibodies against a single antigen, and each T cell is capable of attaching to only one type of foreign antigen.
If each lymphocyte is specific for only one type of antigen, how does an immune response develop? The theory of clonal selection states that, initially, there is a large pool of millions of different lymphocytes, each capable of binding only one antigen (Figure 22.21), so millions of different foreign antigens can be detected. To illustrate clonal selection, let’s imagine that a foreign protein enters the body. Only a few lymphocytes in the pool will be specific for this particular foreign antigen. When one of these lymphocytes encounters the foreign antigen and binds to it, that lymphocyte is stimulated to divide. The lymphocyte proliferates rapidly, producing a large population of genetically identical cells—a clone—each of which is specific for that particular antigen.
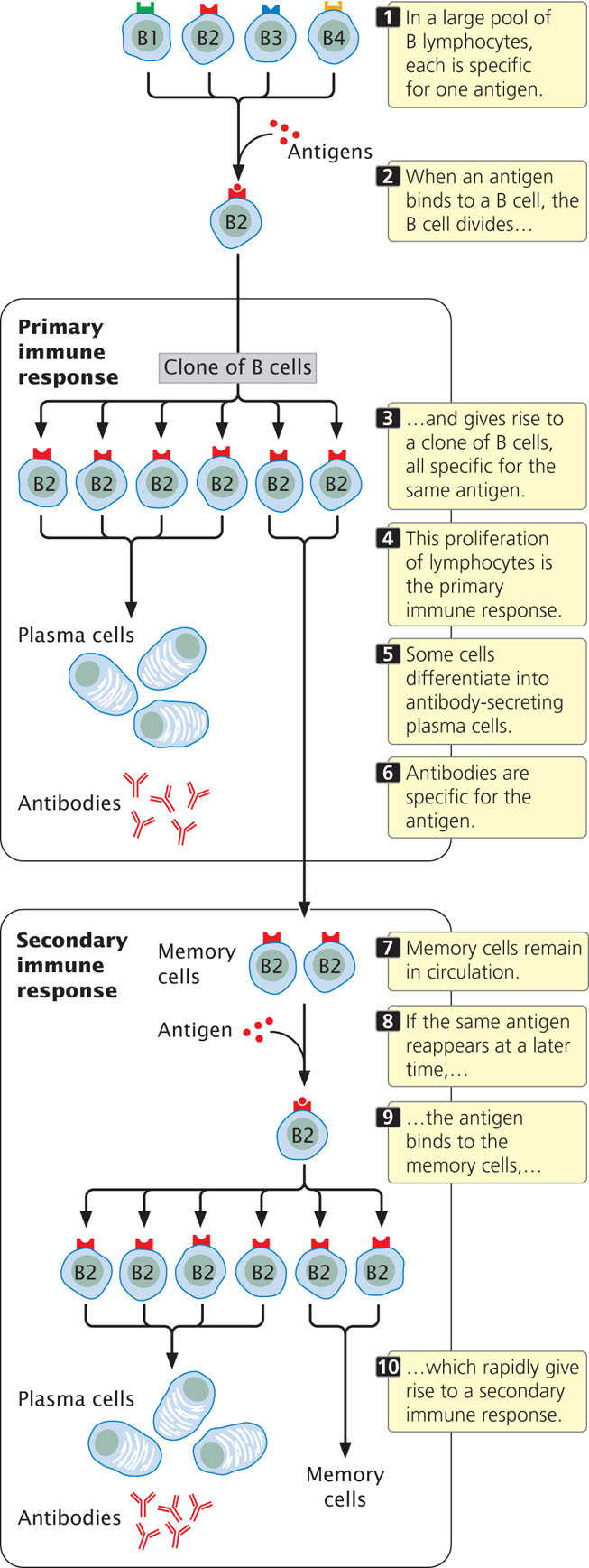
This initial proliferation of antigen-specific B and T cells is known as a primary immune response (see Figure 22.21); in most cases, the primary response destroys the foreign antigen. Following the primary immune response, most of the lymphocytes in the clone die, but a few continue to circulate in the body. These memory cells may remain in circulation for years or even for the rest of a person’s life. Should the same antigen reappear at some time in the future, memory cells specific to that antigen become activated and quickly give rise to another clone of cells capable of binding the antigen. The rise of this second clone is termed a secondary immune response (see Figure 22.21). The ability to quickly produce a second clone of antigen-specific cells permits the long-lasting immunity that often follows recovery from a disease. For example, people who have chicken pox usually have life-long immunity to the disease. The secondary immune response is also the basis for vaccination, which stimulates a primary immune response to an antigen and results in memory cells that can quickly produce a secondary response if that same antigen appears in the future. Three sets of proteins are used in immune responses: antibodies, T-cell receptors, and the major histocompatibility antigens. The next section explores how the enormous diversity in these proteins is generated.
651
CONCEPTS
Each B cell and T cell of the immune system is genetically capable of binding one type of foreign antigen. When a lymphocyte binds to an antigen, the lymphocyte undergoes repeated division, giving rise to a clone of genetically identical lymphocytes (the primary response), all of which are specific for that same antigen. Memory cells remain in circulation for long periods of time; if the antigen reappears, the memory cells rapidly proliferate and generate a secondary immune response.
Immunoglobulin Structure
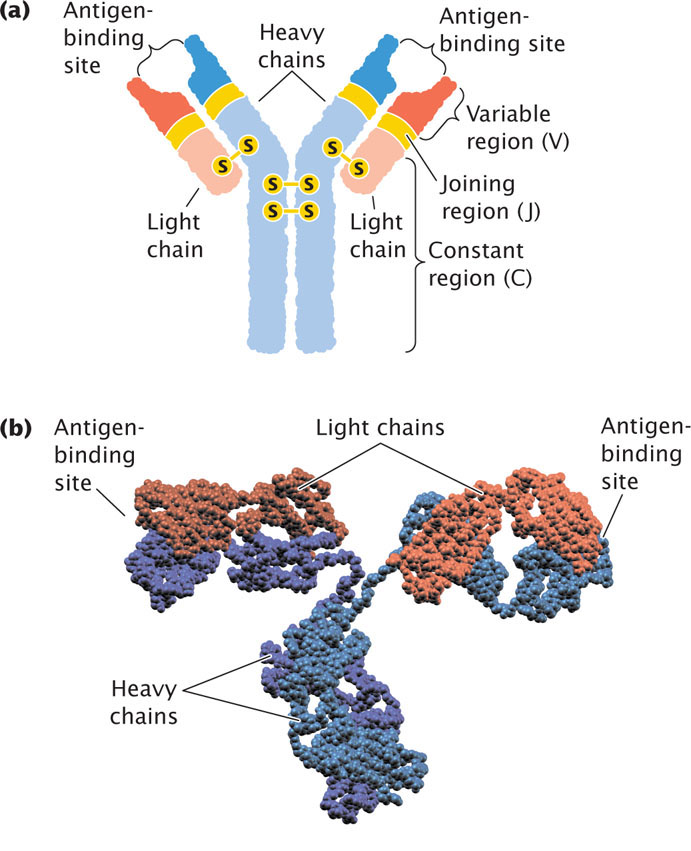
The principal products of the humoral immune response are antibodies—also called immunoglobulins. Each immunoglobulin (Ig) molecule consists of four polypeptide chains—two identical light chains and two identical heavy chains—that form a Y-shaped structure (Figure 22.22). Disulfide bonds link the two heavy chains in the stem of the Y and attach a light chain to a heavy chain in each arm of the Y. Binding sites for antigens are at the ends of the two arms.
652
The light chains of an immunoglobulin are of two basic types: kappa chains and lambda chains. An immunoglobulin molecule can have two kappa chains or two lambda chains, but it cannot have one of each type. Both the light and the heavy chains have a variable region at one end and a constant region at the other end; the variable regions of different immunoglobulin molecules vary in amino acid sequence, whereas the constant regions of different immunoglobulins are similar in sequence. The variable regions of both light and heavy chains make up the antigen-binding regions and specify the type of antigen to which the antibody can bind.
The Generation of Antibody Diversity
The immune system is capable of making antibodies against virtually any antigen that might be encountered in a person’s lifetime: each person is capable of making about 1015 different antibody molecules. Antibodies are proteins, so the amino acid sequences of all 1015 potential antibodies must be encoded in the human genome. However, there are fewer than 1 × 105 genes in the human genome and, in fact, only 3 × 109 total base pairs—so how can this huge diversity of antibodies be encoded?
The answer lies in the fact that antibody genes are composed of segments. There are a number of copies of each type of segment, each differing slightly from the others. In the maturation of a lymphocyte, the segments are joined together to create an immunoglobulin gene. The particular copy of each segment used is random and, because there are multiple copies of each type, there are many possible combinations of the segments. A limited number of segments can therefore encode a huge diversity of antibodies.
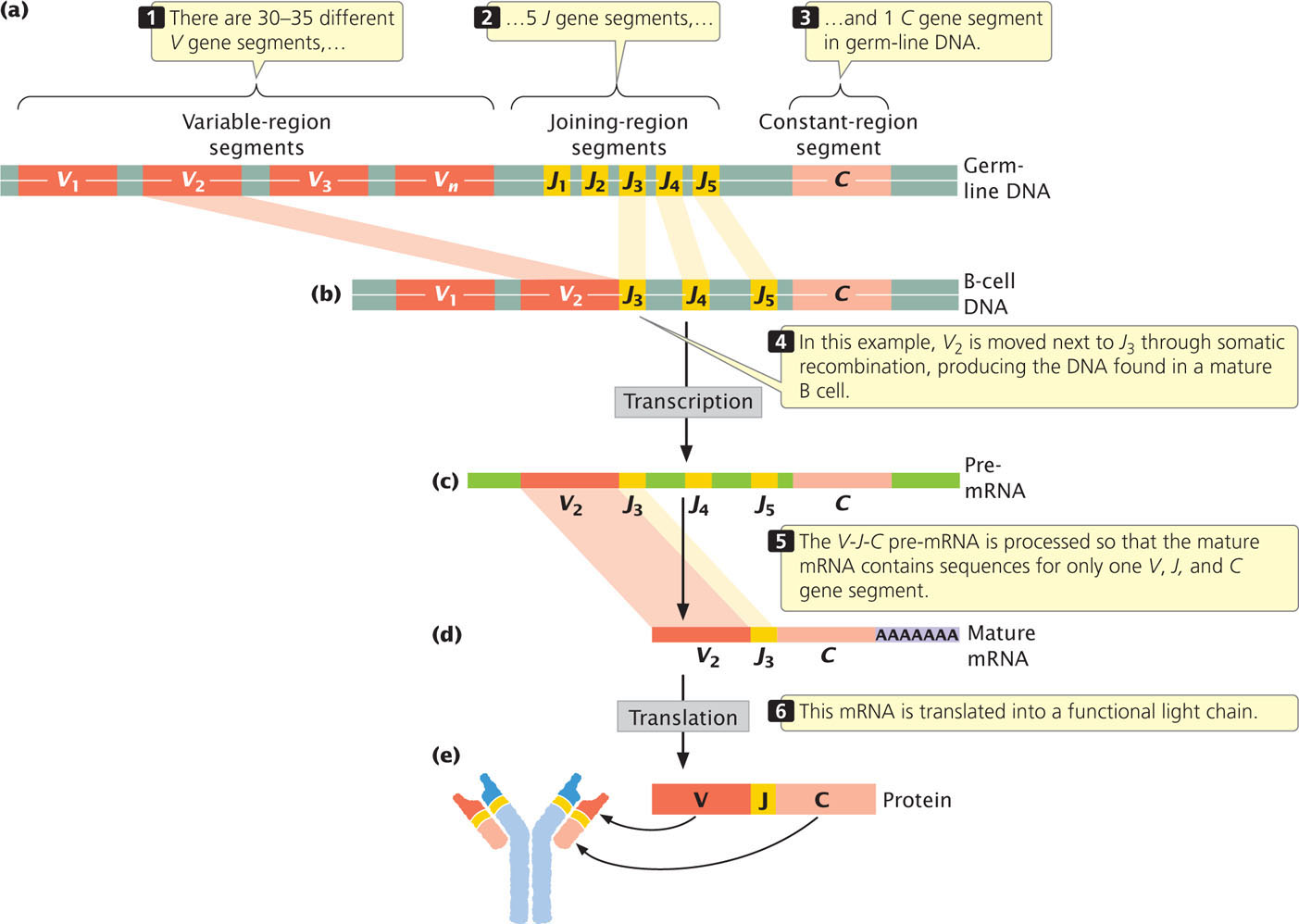
To illustrate this process of antibody assembly, let’s consider the immunoglobulin light chains. Kappa and lambda chains are encoded by separate genes on different chromosomes. Each gene is composed of three types of segments: V, for variable; J, for joining; and C, for constant. The V segments encode most of the variable region of the light chains, the C segment encodes the constant region of the chain, and the J segments encode a short set of nucleotides that join the V and C segments together. The number of V, J, and C segments differs among species. For the human kappa gene, there are from 30 to 35 different functional V gene segments, 5 different J genes, and a single C gene segment, all of which are present in the germ-line DNA (Figure 22.23a). The V gene segments, which are about 400 bp in length, are located on the same chromosome and are separated from one another by about 7000 bp. The J gene segments are about 30 bp in length and all together encompass about 1400 bp.
653
Initially, an immature lymphocyte inherits all of the V gene segments and all of the J gene segments present in the germ line. In the maturation of the lymphocyte, somatic recombination within a single chromosome moves one of the V gene segments to a position next to one of the J gene segments (Figure 22.23b). In Figure 22.23b, V2 (the second of approximately 35 different V gene segments) undergoes somatic recombination, which places it next to J3 (the third of 5 J gene segments); the intervening segments are lost.
After somatic recombination has taken place, the light chain gene is transcribed into pre-mRNA that contains one V gene and several J genes, along with the C gene (Figure 22.23c). The resulting pre-mRNA is processed (Figure 22.23d) to produce a mature mRNA that contains only sequences for a single V, J, and C segment; this mRNA is translated into a functional light chain (Figure 22.23e). In this way, each mature human B cell produces a unique type of kappa light chain, and different B cells produce slightly different kappa chains, depending on the combination of V and J segments that are joined together.
The gene that encodes the lambda light chain is organized in a similar way but differs from the kappa gene in the number of copies of the different segments. Somatic recombination takes place among the segments in the same way as that in the kappa gene, generating many possible combinations of lambda light chains. The gene that encodes the immunoglobulin heavy chain also is arranged in V, J, and C segments, but this gene possesses D (for diversity) segments as well. Thus, many different types of light and heavy chains are possible.
Somatic recombination is brought about by RAG1 and RAG2 proteins, which generate double-strand breaks at specific nucleotide sequences called recombination signal sequences that flank the V, D, J, and C gene segments. DNA-repair proteins then process and join the ends of particular segments together.
In addition to somatic recombination, other mechanisms add to antibody diversity. First, each type of light chain can potentially combine with each type of heavy chain to make a functional immunoglobulin molecule, increasing the amount of possible variation in antibodies. Second, the recombination process that joins V, J, D, and C gene segments in the developing B cell is imprecise, and a few random nucleotides are frequently lost or gained at the junctions of the recombining segments. This junctional diversity greatly enhances variation among antibodies. A third mechanism that adds to antibody diversity is somatic hypermutation, a process that leads to a high mutation rate in the antibody genes. This process is initiated when cytosine bases are deaminated, converting them into uracil. The uracil bases are detected and replaced by DNA-repair mechanisms (see Chapter 18) that are error prone and often replace the original cytosine with a different base, leading to a mutation.
Through the processes of somatic recombination, junctional diversity, and somatic hypermutation, each lymphocyte comes to possess a unique set of genetic information (different from that in other lymphocytes) that encodes an antibody specific to a particular antigen.  TRY PROBLEM 28
TRY PROBLEM 28
CONCEPTS
The genes encoding the antibody chains are organized in segments, and germ-line DNA contains multiple versions of each segment. The many possible combinations of V, J, and D segments permit an immense variety of different antibodies to be generated. This diversity is augmented by the different combinations of light and heavy chains, the random addition and deletion of nucleotides at the junctions of the segments, and the high mutation rates in the immunoglobulin genes.
 CONCEPT CHECK 7
CONCEPT CHECK 7
How does somatic recombination differ from alternative splicing of RNA?
T-Cell-Receptor Diversity
Like B cells, each mature T cell has genetically determined specificity for one type of antigen that is mediated through the cell’s receptors. T-cell receptors are structurally similar to immunoglobulins (Figure 22.24) and are located on the cell surface; most T-cell receptors are composed of one alpha and one beta polypeptide chain held together by disulfide bonds. One end of each chain is embedded in the cell membrane; the other end projects away from the cell and binds antigens. Like the immunoglobulin chains, each chain of the T-cell receptor possesses a constant region and a variable region (see Figure 22.22); the variable regions of the two chains provide the antigen-binding site.
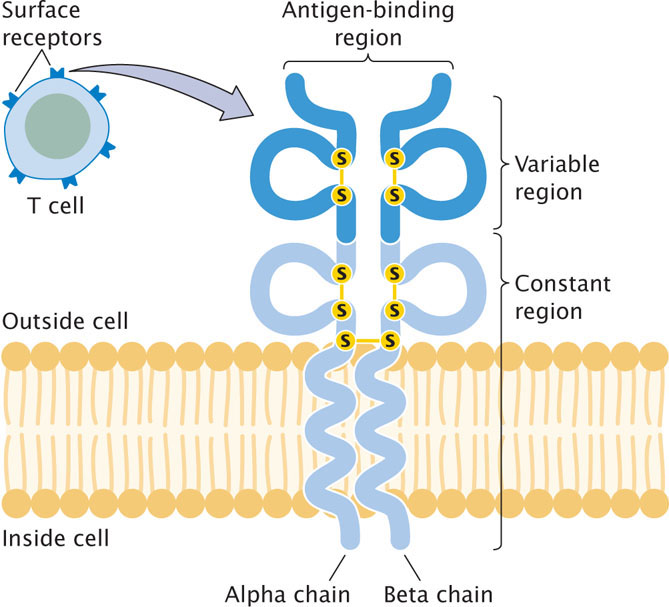
The genes that encode the alpha and beta chains of the T-cell receptor are organized much like those that encode the heavy and light chains of immunoglobulins: each gene is made up of segments that undergo somatic recombination before the gene is transcribed. For example, the human gene for the alpha chain initially consists of 44 to 46 V gene segments, 50 J gene segments, and a single C gene segment. The organization of the gene for the beta chain is similar, except that it also contains D gene segments. Alpha and beta chains combine randomly and there is junctional diversity, but there is no evidence for somatic hypermutation in T-cell-receptor genes.
654
CONCEPTS
Like the genes that encode antibodies, the genes for the T-cell-receptor chains consist of segments that undergo somatic recombination, generating an enormous diversity of antigen-binding sites.
Major Histocompatibility Complex Genes
When tissues are transferred from one species to another or even from one member to another within a species, the transplanted tissues are usually rejected by the host animal. The results of early studies demonstrated that this graft rejection is due to an immune response that takes place when antigens on the surface of cells of the grafted tissue are detected and attacked by T cells in the host organism. The antigens that elicit graft rejection are referred to as histocompatibility antigens, and they are encoded by a cluster of genes called the major histocompatibility complex (MHC).
T cells are activated only when the T-cell receptor simultaneously binds both a foreign antigen and the host cell’s own histocompatibility antigen. The reason for this requirement is not clear; it may reserve T cells for action against pathogens that have invaded cells. When a foreign body, such as a virus, is ingested by a macrophage or other cell, partly digested pieces of the foreign body containing antigens are displayed on the cell’s surface (Figure 22.25). A cell infected with a virus may also express viral antigens on its cell surface. Through their T-cell receptors, T cells bind to both the histocompatibility protein and the foreign antigen and secrete substances that either destroy the antigen-containing cell, activate other B and T cells, or do both.
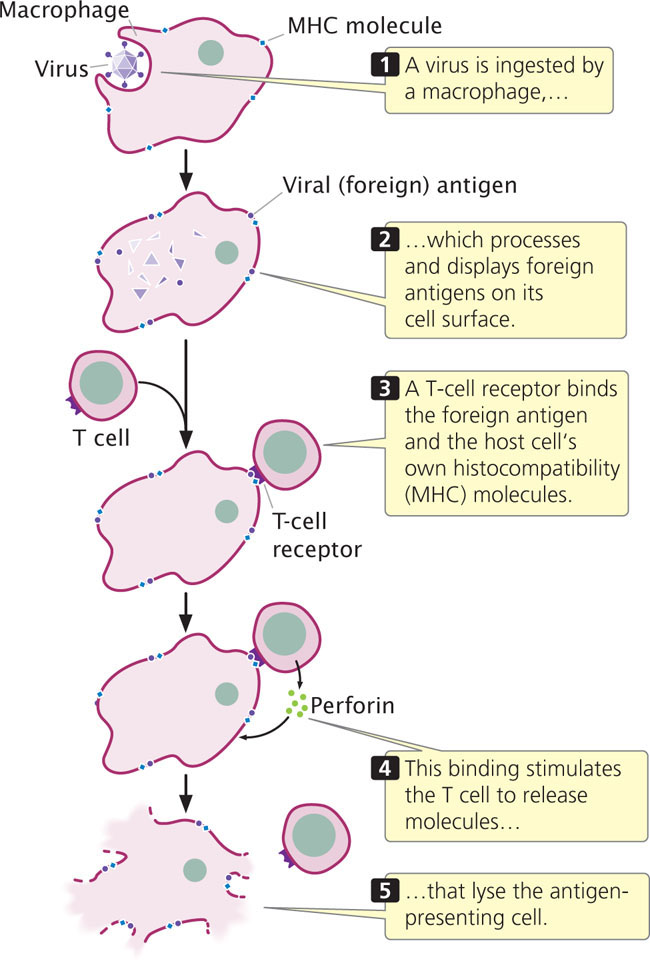
The MHC genes are among the most variable genes known: there are more than 100 different alleles for some MHC loci. Because each person possesses five or more MHC loci and because many alleles are possible at each locus, no two people (with the exception of identical twins) produce the same set of histocompatibility antigens. The variation in histocompatibility antigens provides each of us with a unique identity for our own cells, which allows our immune systems to distinguish self from nonself. This variation is also the cause of rejection in organ transplants.
CONCEPTS
The MHC genes encode proteins that provide identity to the cells of each individual organism. To bring about an immune response, a T-cell receptor must simultaneously bind both a histocompatibility (self) antigen and a specific foreign antigen.
Genes and Organ Transplants
For a person with a seriously impaired organ, a transplant operation may offer the only hope of survival. Successful transplantation requires more than the skills of a surgeon; it also requires a genetic match between the patient and the person donating the organ. The fate of transplanted tissue depends largely on the type of antigens present on the surface of its cells. Because foreign tissues are usually rejected by the host, the successful transplantation of tissues between different persons is very difficult. Tissue rejection can be partly inhibited by drugs that interfere with cellular immunity. Unfortunately, this treatment can create serious problems for transplant patients because they may have difficulty fighting off common pathogens and may thus die of infection. The only other option for controlling the immune reaction is to carefully match the donor and the recipient, maximizing the genetic similarities.
The tissue antigens that elicit the strongest immune reaction are the very ones used by the immune system to mark its own cells: those encoded by the major histocompatibility complex. The MHC spans a region of more than 3 million base pairs on human chromosome 6 and has many alleles, providing different MHC antigens on the cells of different people and allowing the immune system to recognize foreign cells. The severity of an immune rejection of a transplanted organ depends on the number of mismatched MHC antigens on the cells of the transplanted tissue. The ABO red-blood-cell antigens also are important because they elicit a strong immune reaction. The ideal donor is the patient’s own identical twin, who will have exactly the same MHC and ABO antigens. Unfortunately, most patients don’t have an identical twin. The next-best donor is a sibling with the same major MHC and ABO antigens. If a sibling is not available, donors from the general population are considered. An attempt is made to match as many of the MHC antigens of the donor and recipient as possible, and immunosuppressive drugs are used to control rejection due to the mismatches. The long-term success of transplants depends on the closeness of the match. Survival rates after kidney transplants (the most successful of the major organ transplants) increase from 63% with zero or one MHC match to 90% with four matches.
655
Scientists have now been successful in inducing adult cells to lose their specialized characteristics and return to an undifferentiated state called a pluripotent stem cell (see Chapter 21), which is capable of developing into many different cell types. In the future it may be possible to create pluripotent stem cells from a person’s adult cells, and then grow those cells into tissues or organs that could be transplanted back into the same person. Such cells would have the same MHC antigens as the original cell, avoiding immune rejection that occurs with transplants between different people.