4.3 Dosage Compensation Equalizes the Amount of Protein Produced by X-Linked and Autosomal Genes in Some Animals
In species with XX-XY sex determination, differences in the number of X chromosomes possessed by males and females present a special problem in development. In females, there are two copies of the X chromosome and two copies of each autosome, so genes on the X chromosomes and on autosomes are “in balance.” In males, however, there is only a single X chromosome, while there are two copies of every autosome. Because the amount of protein produced is often a function of the number of gene copies encoding the protein, in males there is likely to be less protein encoded by X-linked genes than protein encoded by autosomal genes. This difference can be detrimental, because protein concentration often plays a critical role in development.
93
Some animals have overcome this problem by evolving mechanisms to equalize the amount of protein produced by the single X and two autosomes in the heterogametic sex. These mechanisms are referred to as dosage compensation. In fruit flies, dosage compensation is achieved by a doubling of the activity of the genes on the X chromosome of males but not of females. In placental mammals, the expression of dosage-sensitive genes on X chromosomes of both males and females has increased, coupled with inactivation of one of the X chromosomes in females, so that expression of X-linked and autosomal genes is balanced in both males and females.
For unknown reasons, the presence of sex chromosomes does not always produce problems of gene dosage, and dosage compensation of X-linked genes is not universal. A number of animals do not exhibit obvious mechanisms of dosage compensation. This includes butterflies and moths, birds, some fish, and even the duckbill platypus. As we will discuss in the next section, even in placental mammals a number of genes escape dosage compensation.
Lyon Hypothesis
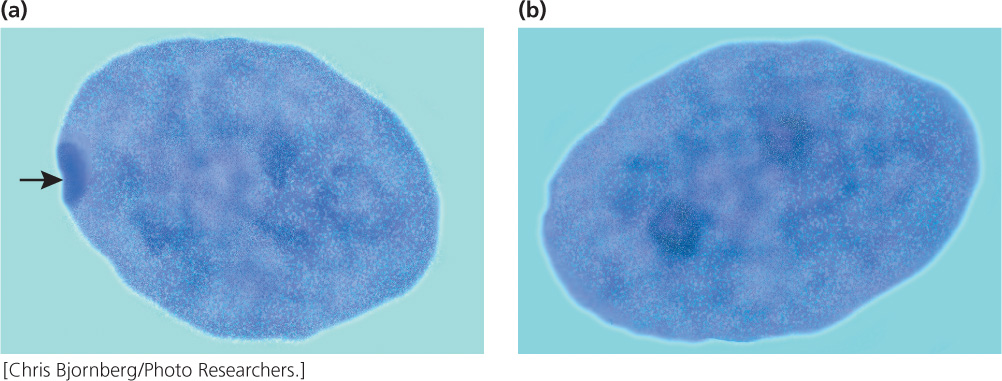
In 1949, Murray Barr observed condensed, darkly staining bodies in the nuclei of cells from female cats (Figure 4.17); these structures became known as Barr bodies. Mary Lyon proposed in 1961 that the Barr body was an inactive X chromosome; her hypothesis (now generally accepted for placental mammals) has become known as the Lyon hypothesis. She suggested that, within each female cell, one of the two X chromosomes becomes inactive; which X chromosome is inactivated is random. If a cell contains more than two X chromosomes, all but one of them are inactivated. The number of Barr bodies present in human cells with different complements of sex chromosomes is shown in Table 4.3.
| Sex Chromosomes | Syndrome | Number of Barr Bodies |
|---|---|---|
| XX | None | 1 |
| XY | None | 0 |
| XO | Turner | 0 |
| XXY | Klinefelter | 1 |
| XXYY | Klinefelter | 1 |
| XXXY | Klinefelter | 2 |
| XXXXY | Klinefelter | 3 |
| XXX | Triple-X | 2 |
| XXXX | Poly-X female | 3 |
| XXXXX | Poly-X female | 4 |
As a result of X inactivation, females of placental mammals are functionally hemizygous at the cellular level for X-linked genes. In females that are heterozygous at an X-linked locus, approximately 50% of the cells will express one allele and 50% will express the other allele; thus, in heterozygous females, proteins encoded by both alleles are produced, although not within the same cell. This functional hemizygosity means that cells in females are not identical with respect to the expression of the genes on the X chromosome; females are mosaics for the expression of X-linked genes.
Random X inactivation takes place early in development—in humans, within the first few weeks of development. After an X chromosome has become inactive in a cell, it remains inactive and is inactive in all somatic cells that descend from the cell. Thus, neighboring cells tend to have the same X chromosome inactivated, producing a patchy pattern (mosaic) for the expression of an X-linked characteristic in heterozygous females.
This patchy distribution can be seen in tortoiseshell and calico cats (Figure 4.18). Although many genes contribute to coat color and pattern in domestic cats, a single X-linked locus determines the presence of orange color. There are two possible alleles at this locus: X+, which produces non-orange (usually black) fur, and Xo, which produces orange fur. Males are hemizygous and thus may be black (X+Y) or orange (Xo Y) but not black and orange. (Rare tortoiseshell males can arise from the presence of two X chromosomes, X+Xo Y.) Females may be black (X+X+), orange (Xo Xo), or tortoiseshell (X+Xo), the tortoiseshell pattern arising from a patchy mixture of black and orange fur. Each orange patch is a clone of cells derived from an original cell in which the black allele is inactivated, and each black patch is a clone of cells derived from an original cell in which the orange allele is inactivated.

94
The Lyon hypothesis suggests that the presence of variable numbers of X chromosomes should not affect the phenotype in mammals, because any X chromosomes in excess of one X chromosome should be inactivated. However, persons with Turner syndrome (XO) differ from XX females, and those with Klinefelter syndrome (XXY) differ from XY males. How do these conditions arise in the face of dosage compensation?
These disorders probably arise because some X-linked genes escape inactivation. Indeed, the nature of X inactivation is more complex than originally envisioned. Studies of individual genes now reveal that only about 75% of X-linked human genes are permanently inactivated. About 15% completely escape X inactivation, meaning that these genes produce twice as much protein in females as they do in males. The remaining 10% are inactivated in some females but not in others. The reason for this variation among females in the activation of some X-linked genes is not known. Furthermore, recent research indicates that X-inactivation does not actually equalize dosage of many X-linked and autosomal genes in humans and mice.  TRY PROBLEM 44
TRY PROBLEM 44
Mechanism of Random X Inactivation
Random inactivation of X chromosomes requires two steps. In the first step, the cell somehow assesses, or counts, how many X chromosomes are present. In the second step, one X chromosome is selected to become the active X chromosome and all others are silenced.
Although many details of X-chromosome inactivation remain unknown, several genes and sequences that participate in the process have been identified. Foremost among them is a gene called Xist (for X-inactivation-specific transcript). On the X chromosomes destined to become inactivated, the Xist gene is active, producing a 17,000 nucleotide long RNA molecule that coats the X chromosome, and leads to inactivation of the genes on it, probably by recruiting protein complexes that alter chromatin structure. On the X chromosome destined to become active, other genes repress the activity of Xist so that the Xist RNA never coats the X chromosome and genes on this chromosome remain active.
CONCEPTS
In placental mammals, all but one X chromosome are inactivated in each cell; which of the X chromosomes is inactivated is random and varies from cell to cell.
 CONCEPT CHECK 8
CONCEPT CHECK 8
How many Barr bodies will a male with XXXYY chromosomes have in each of his cells?