5.3 Sex Influences the Inheritance and Expression of Genes in a Variety of Ways
In Chapter 4, we considered characteristics encoded by genes located on the sex chromosomes (sex-linked traits) and how their inheritance differs from the inheritance of traits encoded by autosomal genes. X-linked traits, for example, are passed from father to daughter but never from father to son, and Y-linked traits are passed from father to all sons. Now, we will examine additional influences of sex, including the effect of the sex of an individual organism on the expression of genes on autosomal chromosomes, on characteristics determined by genes located in the cytoplasm, and on characteristics for which the genotype of only the maternal parent determines the phenotype of the offspring. Finally, we will look at situations in which the expression of genes on autosomal chromosomes is affected by the sex of the parent from whom the genes are inherited.
Sex-Influenced and Sex-Limited Characteristics
Sex-influenced characteristics are determined by autosomal genes and are inherited according to Mendel’s principles, but they are expressed differently in males and females. In this case, a particular trait is more readily expressed in one sex; in other words, the trait has higher penetrance in one of the sexes.
120
For example, the presence of a beard on some goats is determined by an autosomal gene (Bb) that is dominant in males and recessive in females. In males, a single allele is required for the expression of this trait: both the homozygote (BbBb) and the heterozygote (BbB+) have beards, whereas the B+B+ male is beardless.
| Genotype | Males | Females |
|---|---|---|
| B+B+ | beardless | beardless |
| B+Bb | bearded | beardless |
| BbBb | bearded | bearded |
In contrast, females require two bearded alleles in order for this trait to be expressed: the homozygote BbBb has a beard, whereas the heterozygote (BbB+) and the other homozygote (B+B+) are beardless.
The key to understanding the expression of the bearded gene is to look at the heterozygote. In males (for which the presence of a beard is dominant), the heterozygous genotype produces a beard but, in females (for which the absence of a beard is dominant), the heterozygous genotype produces a goat without a beard.
Figure 5.12a illustrates a cross between a beardless male (B+B+) and a bearded female (BbBb). The alleles separate into gametes according to Mendel’s principle of segregation, and all the F1 are heterozygous (B+Bb). Because the trait is dominant in males and recessive in females, all the F1 males will be bearded and all the F1 females will be beardless. When the F1 are crossed with one another, 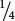 of the F2 progeny are BbBb,
of the F2 progeny are BbBb, 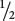 are BbB+, and
are BbB+, and 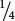 are B+B+ (Figure 5.12b). Because male heterozygotes are bearded,
are B+B+ (Figure 5.12b). Because male heterozygotes are bearded, 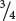 of the males in the F2 possess beards; because female heterozygotes are beardless, only
of the males in the F2 possess beards; because female heterozygotes are beardless, only 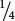 of the females in the F2 are bearded.
of the females in the F2 are bearded.
An extreme form of sex-influenced inheritance, a sex-limited characteristic is encoded by autosomal genes that are expressed in only one sex; the trait has zero penetrance in the other sex. In domestic chickens, some males display a plumage pattern called cock feathering (Figure 5.13a). Other males and all females display a pattern called hen feathering (Figure 5.13b and c). Cock feathering is an autosomal recessive trait that is sex-limited to males. Because the trait is autosomal, the genotypes of males and females are the same, but the phenotypes produced by these genotypes differ in males and females:
| Genotype | Male phenotype | Female phenotype |
|---|---|---|
| HH | hen feathering | hen feathering |
| Hh | hen feathering | hen feathering |
| hh | cock feathering | hen feathering |

121
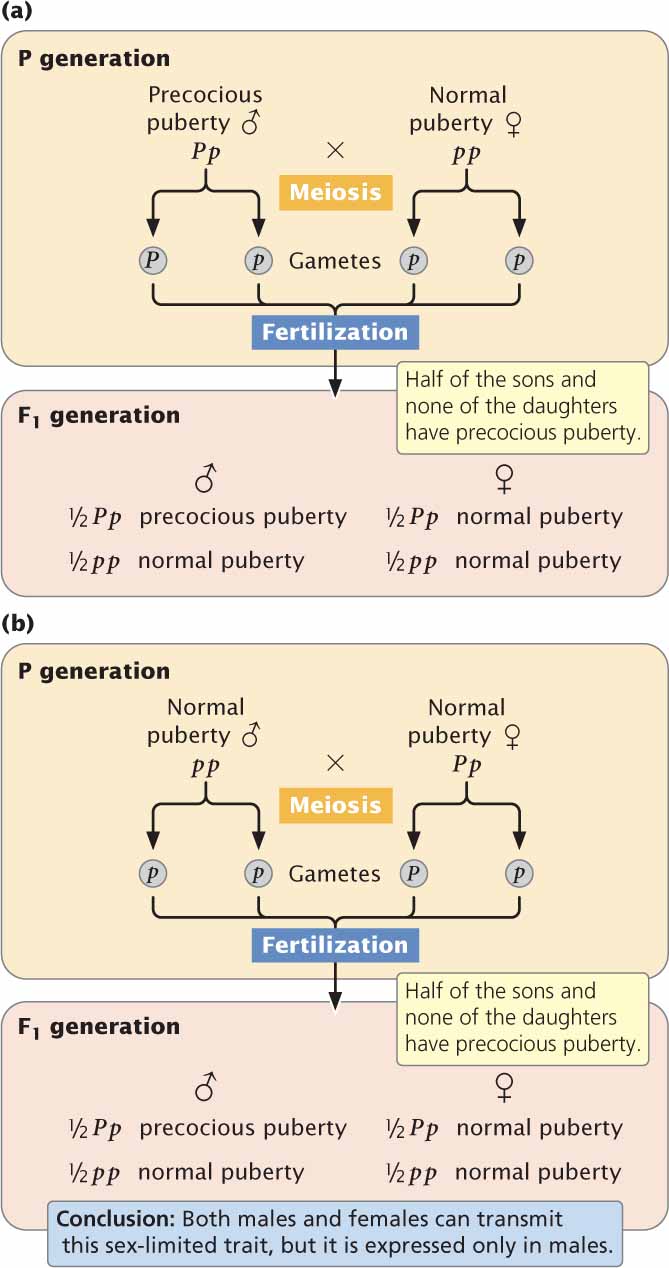
An example of a sex-limited characteristic in humans is male-limited precocious puberty. There are several types of precocious puberty in humans, most of which are not genetic. Male-limited precocious puberty, however, results from an autosomal dominant allele (P) that is expressed only in males; females with the gene are normal in phenotype. Males with precocious puberty undergo puberty at an early age, usually before the age of 4. At this time, the penis enlarges, the voice deepens, and pubic hair develops. There is no impairment of sexual function; affected males are fully fertile. Most are short as adults because the long bones stop growing after puberty.
Because the trait is rare, affected males are usually heterozygous (Pp). A male with precocious puberty who mates with a woman who has no family history of this condition will transmit the allele for precocious puberty to 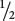 of their children (Figure 5.14a), but it will be expressed only in the sons. If one of the heterozygous daughters (Pp) mates with a male who has normal puberty (pp),
of their children (Figure 5.14a), but it will be expressed only in the sons. If one of the heterozygous daughters (Pp) mates with a male who has normal puberty (pp), 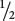 of their sons will exhibit precocious puberty (Figure 5.14b). Thus a sex-limited characteristic can be inherited from either parent, although the trait appears in only one sex.
of their sons will exhibit precocious puberty (Figure 5.14b). Thus a sex-limited characteristic can be inherited from either parent, although the trait appears in only one sex.  TRY PROBLEM 35
TRY PROBLEM 35
CONCEPTS
Sex-influenced characteristics are encoded by autosomal genes that are more readily expressed in one sex. Sex-limited characteristics are encoded by autosomal genes whose expression is limited to one sex.
 CONCEPT CHECK 9
CONCEPT CHECK 9
How do sex-influenced and sex-limited characteristics differ from sex-linked characteristics?
Cytoplasmic Inheritance
Mendel’s principles of segregation and independent assortment are based on the assumption that genes are located on chromosomes in the nucleus of the cell. For most genetic characteristics, this assumption is valid, and Mendel’s principles allow us to predict the types of offspring that will be produced in a genetic cross. However, not all the genetic material of a cell is found in the nucleus; some characteristics are encoded by genes located in the cytoplasm. These characteristics exhibit cytoplasmic inheritance.
122
A few organelles, notably chloroplasts and mitochondria, contain DNA. The human mitochondrial genome contains about 15,000 nucleotides of DNA, encoding 37 genes. Compared with that of nuclear DNA, which contains some 3 billion nucleotides encoding some 20,000 genes, the size of the mitochondrial genome is very small; nevertheless, mitochondrial and chloroplast genes encode some important characteristics. The molecular details of this extranuclear DNA are discussed in Chapter 11; here, we will focus on patterns of cytoplasmic inheritance.
Cytoplasmic inheritance differs from the inheritance of characteristics encoded by nuclear genes in several important respects. A zygote inherits nuclear genes from both parents; but, typically, all its cytoplasmic organelles, and thus all its cytoplasmic genes, come from only one of the gametes, usually the egg. A sperm from the male parent generally contributes only a set of nuclear genes. Thus most cytoplasmically inherited traits are present in both males and females and are passed from mother to offspring, never from father to offspring. Reciprocal crosses, therefore, give different results when cytoplasmic genes encode a trait. In a few organisms, however, cytoplasmic genes are inherited from the male parent only or from both parents.
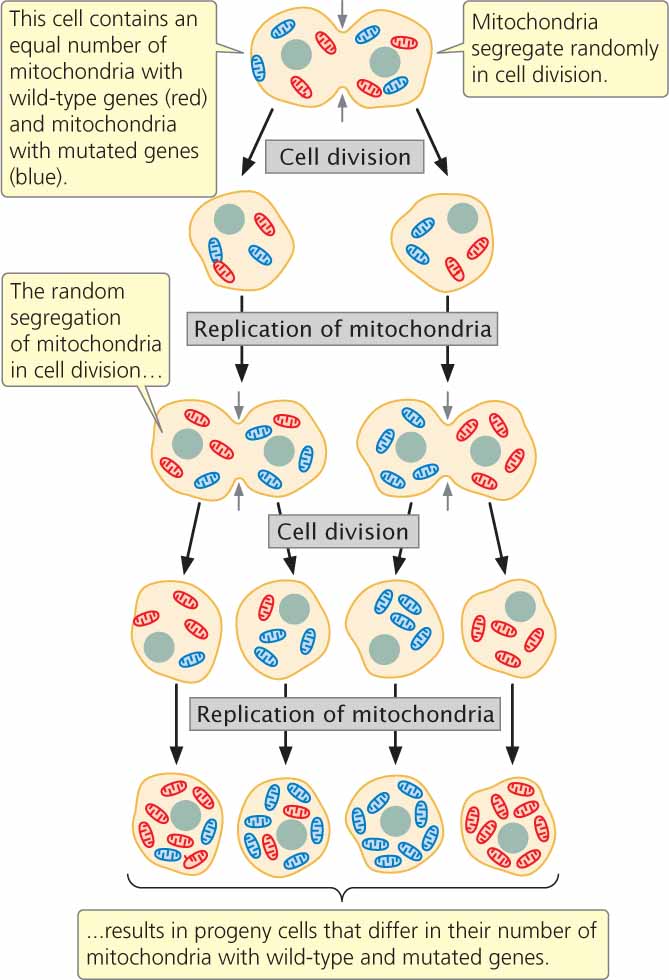
Cytoplasmically inherited characteristics frequently exhibit extensive phenotypic variation because no mechanism analogous to mitosis or meiosis ensures that cytoplasmic genes are evenly distributed in cell division. Thus, different cells and individual offspring will contain various proportions of cytoplasmic genes.
Consider mitochondrial genes. Most cells contain thousands of mitochondria, and each mitochondrion contains from 2 to 10 copies of mitochondrial DNA (mtDNA). Suppose that half of the mitochondria in a cell contain a normal wild-type copy of mtDNA and the other half contain a mutated copy (Figure 5.15). In cell division, the mitochondria segregate into progeny cells at random. Just by chance, one cell may receive mostly mutated mtDNA and another cell may receive mostly wild-type mtDNA. In this way, different progeny from the same mother and even cells within an individual offspring may vary in their phenotypes. Traits encoded by chloroplast DNA (cpDNA) are similarly variable. The characteristics that cytoplasmically inherited traits exhibit are summarized in Table 5.4.
| 1. | Present in males and females. |
| 2. | Usually inherited from one parent, typically the maternal parent. |
| 3. | Reciprocal crosses give different results. |
| 4. | Exhibit extensive phenotypic variation, even within a single family. |
Variegation in Four-O’ClocksIn 1909, cytoplasmic inheritance was recognized by Carl Correns as an exception to Mendel’s principles. Correns, one of the biologists who rediscovered Mendel’s work, studied the inheritance of leaf variegation in the four-o’clock plant, Mirabilis jalapa. Correns found that the leaves and shoots of one variety of four-o’clock were variegated, displaying a mixture of green and white splotches. He also noted that some branches of the variegated strain had all-green leaves; other branches had all-white leaves. Each branch produced flowers; so Correns was able to cross flowers from variegated, green, and white branches in all combinations (Figure 5.16). The seeds from green branches always gave rise to green progeny, no matter whether the pollen was from a green, white, or variegated branch. Similarly, flowers on white branches always produced white progeny. Flowers on the variegated branches gave rise to green, white, and variegated progeny, in no particular ratio.
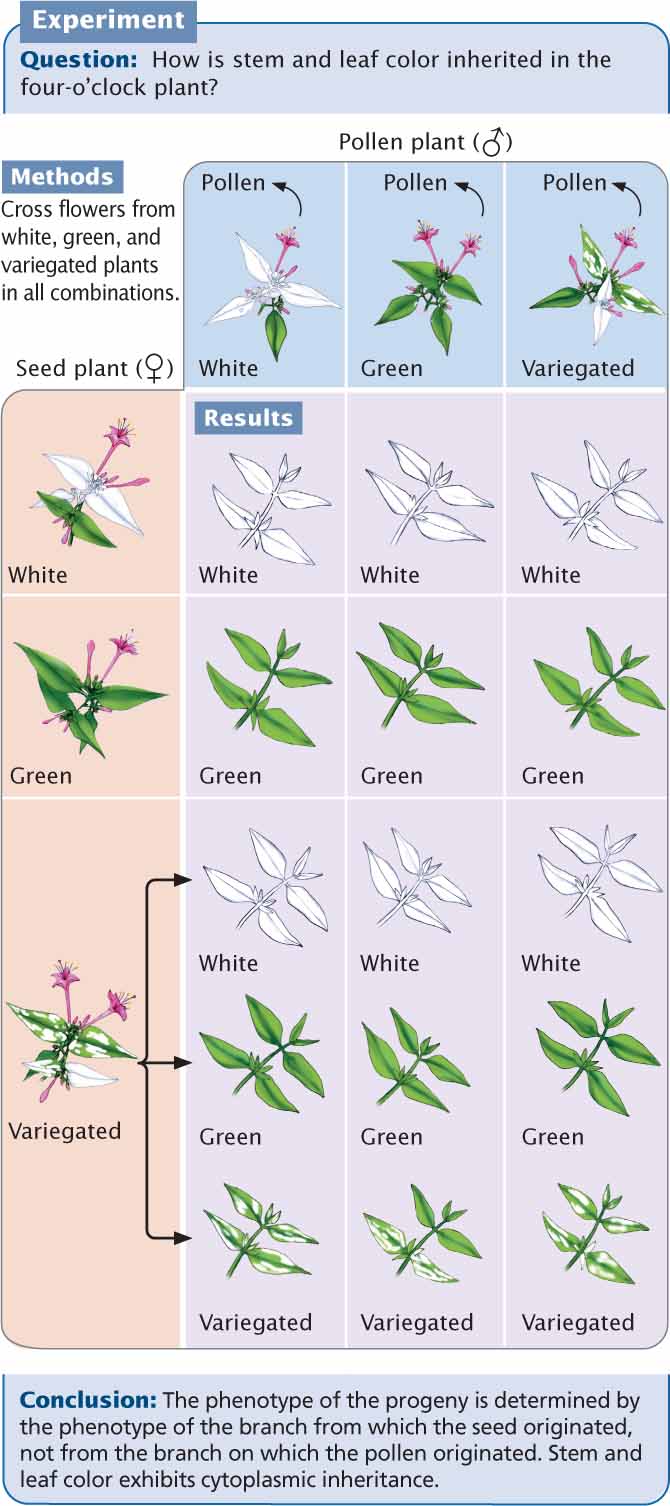
Correns’s crosses demonstrated the cytoplasmic inheritance of variegation in four-o’clocks. The phenotypes of the offspring were determined entirely by the maternal parent, never by the paternal parent (the source of the pollen). Furthermore, the production of all three phenotypes by flowers on variegated branches is consistent with cytoplasmic inheritance. Variegation in these plants is caused by a defective gene in cpDNA, which results in a failure to produce the green pigment chlorophyll. Cells from green branches contain normal chloroplasts only, cells from white branches contain abnormal chloroplasts only, and cells from variegated branches contain a mixture of normal and abnormal chloroplasts. In the flowers from variegated branches, the random segregation of chloroplasts in the course of oogenesis produces some egg cells with normal cpDNA, which develop into green progeny; other egg cells with only abnormal cpDNA develop into white progeny; and, finally, still other egg cells with a mixture of normal and abnormal cpDNA develop into variegated progeny.
123
Mitochondrial DiseasesA number of human diseases (mostly rare) that exhibit cytoplasmic inheritance have been identified. These disorders arise from mutations in mtDNA, most of which occur in genes encoding components of the electron-transport chain, which generates most of the ATP (adenosine triphosphate) in aerobic cellular respiration. One such disease is Leber hereditary optic neuropathy (LHON). Patients who have this disorder experience rapid loss of vision in both eyes, resulting from the death of cells in the optic nerve. This loss of vision typically occurs in early adulthood (usually between the ages of 20 and 24), but it can occur any time after adolescence. There is much clinical variability in the severity of the disease, even within the same family. Leber hereditary optic neuropathy exhibits cytoplasmic inheritance: the trait is passed from mother to all children, sons and daughters alike.
Genetic Maternal Effect
A genetic phenomenon that is sometimes confused with cytoplasmic inheritance is genetic maternal effect, in which the phenotype of the offspring is determined by the genotype of the mother. In cytoplasmic inheritance, the genes for a characteristic are inherited from only one parent, usually the mother. In genetic maternal effect, the genes are inherited from both parents, but the offspring’s phenotype is determined not by its own genotype but by the genotype of its mother.
Genetic maternal effect frequently arises when substances present in the cytoplasm of an egg (encoded by the mother’s nuclear genes) are pivotal in early development. An excellent example is the shell coiling of the snail Lymnaea peregra (Figure 5.17), described in the introduction to this chapter. Right-handed shell coiling is termed dextral, while left-handed coiling is termed sinistral. In Lymnaea peregra the direction of coiling is determined by a pair of alleles; the allele for dextral (s+) is dominant over the allele for sinistral (s). However, the direction of coiling is determined not by that snail’s own genotype, but by the genotype of its mother. The direction of coiling is affected by the way in which the cytoplasm divides soon after fertilization, which in turn is determined by a substance produced by the mother and passed to the offspring in the cytoplasm of the egg.
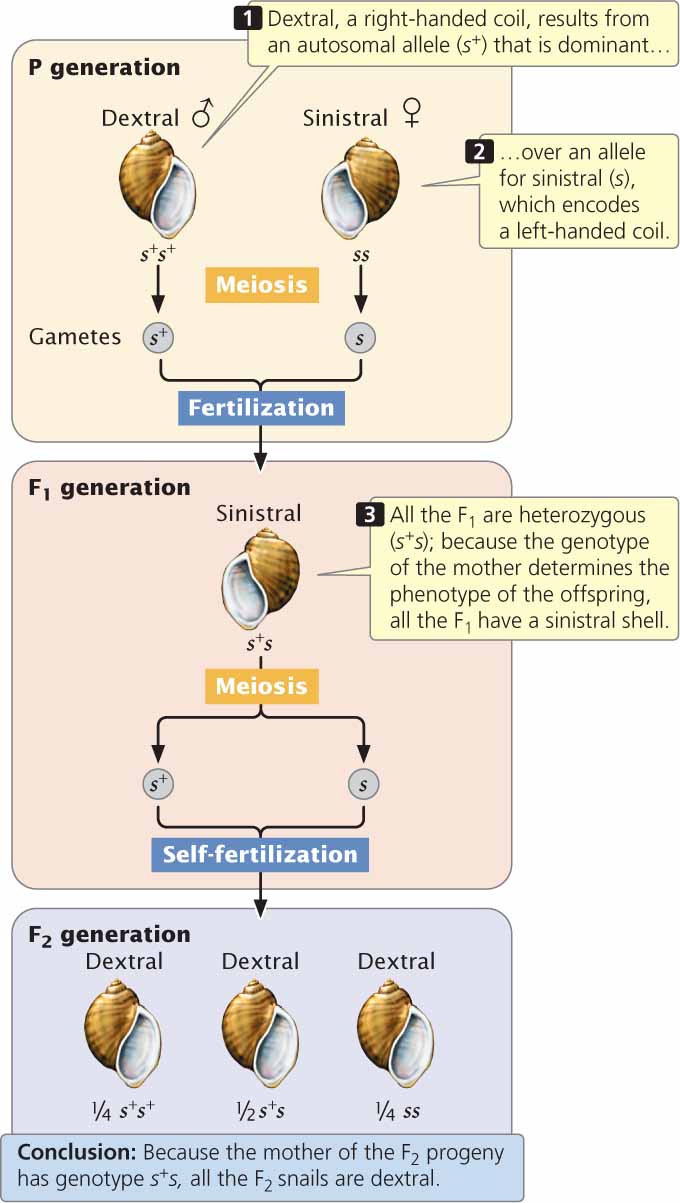
If a male homozygous for dextral alleles (s+s+) is crossed with a female homozygous for sinistral alleles (ss), all of the F1 are heterozygous (s+s) and have a sinistral shell because the genotype of the mother (ss) encodes sinistral coiling (see Figure 5.17). If these F1 snails self-fertilize, the genotypic ratio of the F2 is 1 s+s+ : 2 s+s : 1 ss.
124
Notice that the phenotype of all the F2 snails is dextral coiled, regardless of their genotypes. The F2 offspring are dextral coiled because the genotype of their mother (s+s) encodes a right-coiling shell and determines their phenotype. With genetic maternal effect, the phenotype of the progeny is not necessarily the same as the phenotype of the mother, because the progeny’s phenotype is determined by the mother’s genotype, not her phenotype. Neither the male parent’s nor the offspring’s own genotype has any role in the offspring’s phenotype. However, a male does influence the phenotype of the F2 generation: by contributing to the genotypes of his daughters, he affects the phenotypes of their offspring. Genes that exhibit genetic maternal effects are therefore transmitted through males to future generations. In contrast, genes that exhibit cytoplasmic inheritance are transmitted through only one of the sexes (usually the female).  TRY PROBLEM 39
TRY PROBLEM 39
CONCEPTS
Characteristics exhibiting cytoplasmic inheritance are encoded by genes in the cytoplasm and are usually inherited from one parent, most commonly the mother. In genetic maternal effect, the genotype of the mother determines the phenotype of the offspring.
 CONCEPT CHECK 10
CONCEPT CHECK 10
How might you determine whether a particular trait is due to cytoplasmic inheritance or to genetic maternal effect?
Genomic Imprinting
A basic tenet of Mendelian genetics is that the parental origin of a gene does not affect its expression and, therefore, reciprocal crosses give identical results. We have seen that there are some genetic characteristics—those encoded by X-linked genes and cytoplasmic genes—for which reciprocal crosses do not give the same results. In these cases, males and females do not contribute the same genetic material to the offspring. With regard to autosomal genes, males and females contribute the same number of genes, and paternal and maternal genes have long been assumed to have equal effects. However, the expression of some genes is significantly affected by their parental origin. This phenomenon, the differential expression of genetic material depending on whether it is inherited from the male or female parent, is called genomic imprinting.
A gene that exhibits genomic imprinting in both mice and humans is Igf2, which encodes a protein called insulin-like growth factor 2 (Igf2). Offspring inherit one Igf2 allele from their mother and one from their father. The paternal copy of Igf2 is actively expressed in the fetus and placenta, but the maternal copy is completely silent (Figure 5.18). Both male and female offspring possess Igf2 genes; the key to whether the gene is expressed is the sex of the parent transmitting the gene. In the present example, the gene is expressed only when it is transmitted by a male parent. In other genomically imprinted traits, the trait is expressed only when the gene is transmitted by the female parent. In a way that is not completely understood, the paternal Igf2 allele (but not the maternal allele) promotes placental and fetal growth; when the paternal copy of Igf2 is deleted in mice, a small placenta and low-birth-weight offspring result.
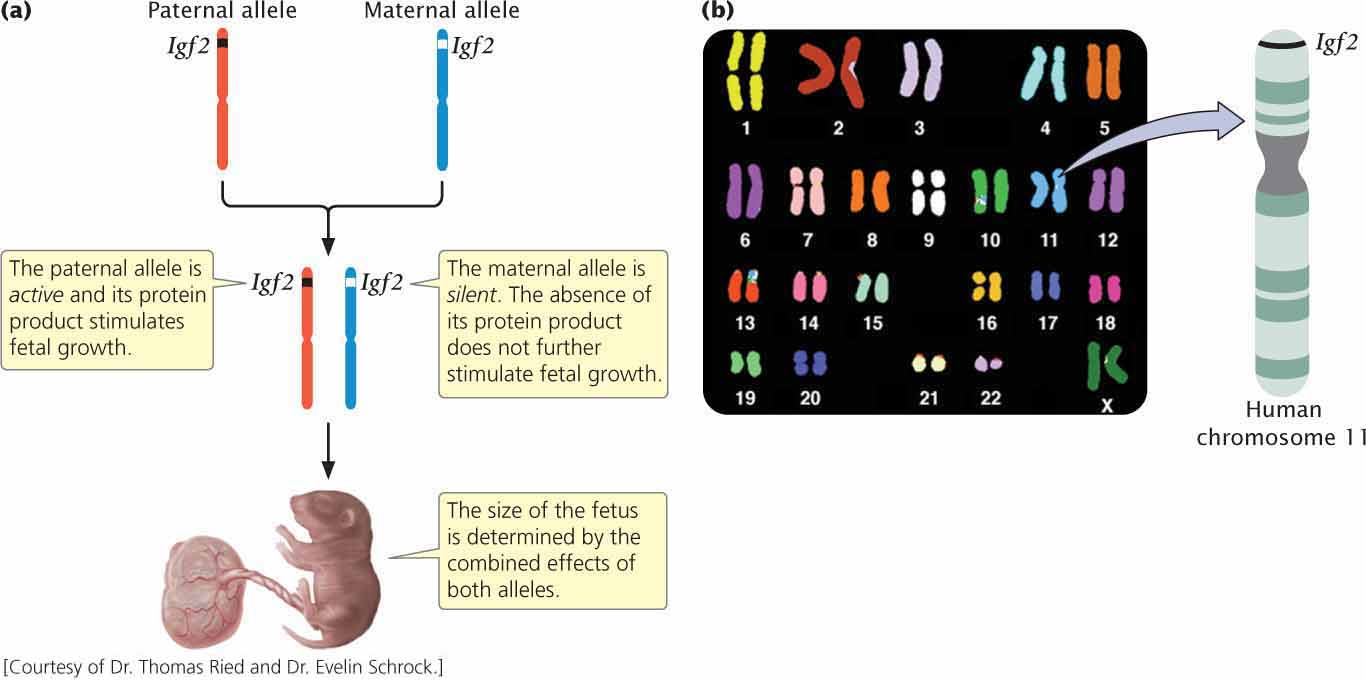
Genomic imprinting has been implicated in several human disorders, including Prader–Willi and Angelman syndromes. Children with Prader–Willi syndrome have small hands and feet, short stature, poor sexual development, and intellectual disability. These children are small at birth and nurse poorly, but as toddlers they develop voracious appetites and frequently become obese. Many persons with Prader–Willi syndrome are missing a small region on the long arm of chromosome 15. The deletion of this region is always inherited from the father. Thus, children with Prader–Willi syndrome lack a paternal copy of genes on the long arm of chromosome 15.
125
The deletion of this same region of chromosome 15 can also be inherited from the mother, but this inheritance results in a completely different set of symptoms, producing Angelman syndrome. Children with Angelman syndrome exhibit frequent laughter, uncontrolled muscle movement, a large mouth, and unusual seizures. They are missing a maternal copy of genes on the long arm of chromosome 15. For normal development to take place, copies of this region of chromosome 15 from both male and female parents are apparently required.
Many imprinted genes in mammals are associated with fetal growth. Imprinting has also been reported in plants, with differential expression of paternal and maternal genes in the endosperm, which, like the placenta in mammals, provides nutrients for the growth of the embryo. The mechanism of imprinting is still under investigation, but the methylation of DNA—the addition of methyl (CH3) groups to DNA nucleotides (see Chapters 10 and 17)—is essential to the process. In mammals, methylation is erased in the germ cells each generation and then reestablished in the course of gamete formation, with sperm and eggs undergoing different levels of methylation, which then causes the differential expression of male and female alleles in the offspring. Some of the different ways in which sex interacts with heredity are summarized in Table 5.5.
| Genetic Phenomenon | Phenotype determined by |
|---|---|
| Sex-linked characteristic | Genes located on the sex chromosome |
| Sex-influenced characteristic | Genes on autosomal chromosomes that are more readily expressed in one sex |
| Sex-limited characteristic | Autosomal genes whose expression is limited to one sex |
| Genetic maternal effect | Nuclear genotype of the maternal parent |
| Cytoplasmic inheritance | Cytoplasmic genes, which are usually inherited entirely from only one parent |
| Genomic imprinting | Genes whose expression is affected by the sex of the transmitting parent |
EpigeneticsGenomic imprinting is just one form of a phenomenon known as epigenetics. Most traits are encoded by genetic information that resides in the sequence of nucleotide bases of DNA—the genetic code, which will be discussed in Chapter 15. However, some traits are caused by alterations to the DNA, such as the addition of methyl groups to some of the DNA bases (DNA methylation), that affect the way in which the DNA sequences are expressed. These changes are often stable and heritable in the sense that they are passed from one cell to another.
126
In genomic imprinting, whether the gene passes through the egg or sperm determines how much methylation of the DNA takes place. The pattern of methylation on a gene is copied when the DNA is replicated and therefore remains on the gene as it is passed from cell to cell through mitosis. However, the pattern of methylation may be modified or removed when the DNA passes through a gamete, and so a gene methylated in sperm may be unmethylated when it is eventually passed down to a daughter’s egg. Ultimately, the amount of methylation determines whether the gene is expressed in the offspring.
These types of reversible changes to DNA that influence the expression of traits are termed epigenetic marks. The inactivation of one of the X chromosomes in female mammals (discussed in Chapter 4) is another type of epigenetic change. We will consider epigenetic changes in more detail in Chapter 21.
CONCEPTS
In genomic imprinting, the expression of a gene is influenced by the sex of the parent transmitting the gene to the offspring. Epigenetic marks are reversible changes in DNA that do not alter the base sequence but may affect how a gene is expressed.
 CONCEPT CHECK 11
CONCEPT CHECK 11
What type of epigenetic mark is responsible for genomic imprinting?