6.4 Genetic Counseling and Genetic Testing Provide Information to Those Concerned about Genetic Diseases and Traits
Our knowledge of human genetic diseases and disorders has expanded rapidly in recent years. The Online Mendelian Inheritance in Man now lists more than 21,000 human genetic diseases, disorders, genes, and traits that have a simple genetic basis. Research has provided a great deal of information about the inheritance, chromosomal location, biochemical basis, and symptoms of many of these genetic traits, diseases, and disorders. This information is often useful to people who have a genetic condition.
151
Genetic Counseling
Genetic counseling is a field that provides information to patients and others who are concerned about hereditary conditions. It is an educational process that helps patients and family members deal with many aspects of a genetic condition including a diagnosis, information about symptoms and treatment, and information about the mode of inheritance. Genetic counseling also helps patients and their families cope with the psychological and physical stress that may be associated with the disorder. Clearly, all of these considerations cannot be handled by a single person; most genetic counseling is done by a team that can include counselors, physicians, medical geneticists, and laboratory personnel. Table 6.3 lists some common reasons for seeking genetic counseling.
| 1. | A person knows of a genetic disease in the family. |
| 2. | A couple has given birth to a child with a genetic disease, birth defect, or chromosomal abnormality. |
| 3. | A couple has a child who is intellectually disabled or has a close relative who is intellectually disabled. |
| 4. | An older woman becomes pregnant or wants to become pregnant. There is disagreement about the age at which a prospective mother who has no other risk factor should seek genetic counseling; many experts suggest that it should be age 35 or older. |
| 5. | Husband and wife are closely related (e.g., first cousins). |
| 6. | A couple experiences difficulties achieving a successful pregnancy. |
| 7. | A pregnant woman is concerned about exposure to an environmental substance (drug, chemical, or virus) that causes birth defects. |
| 8. | A couple needs assistance in interpreting the results of a prenatal or other test. |
| 9. | Both prospective parents are known carriers for a recessive genetic disease or both belong to an ethnic group with a high frequency of a genetic disease. |
Genetic counseling usually begins with a diagnosis of the condition. On the bases of a physical examination, biochemical tests, DNA testing, chromosome analysis, family history, and other information, a physician determines the cause of the condition. An accurate diagnosis is critical, because treatment and the probability of passing the condition on may vary, depending on the diagnosis. For example, there are a number of different types of dwarfism, which may be caused by chromosome abnormalities, single-gene mutations, hormonal imbalances, or environmental factors. People who have dwarfism resulting from an autosomal dominant gene have a 50% chance of passing the condition on to their children, whereas people who have dwarfism caused by a rare recessive gene have a low likelihood of passing the trait on to their children.
When the nature of the condition is known, a genetic counselor meets with the patient and members of the patient’s family and explains the diagnosis. A family pedigree may be constructed, and the probability of transmitting the condition to future generations can be calculated for different family members. The counselor helps the family interpret the genetic risks and explains various available reproductive options, including prenatal diagnosis, artificial insemination, and in vitro fertilization. Often family members have questions about genetic testing that may be available to help determine whether they carry a genetic mutation. The counselor helps them decide whether genetic testing is appropriate and which tests to apply. After the test results are in, the genetic counselor usually helps family members interprete the results.
A family’s decision about future pregnancies frequently depends on the magnitude of the genetic risk, the severity and effects of the condition, the importance of having children, and religious and cultural views. Traditionally, genetic counselors have been trained to apply nondirected counseling, which means that they provide information and facilitate discussion but do not bring their own opinions and values into the discussion. The goal of nondirected counseling is for the family to reach its own decision on the basis of the best available information.
Because of the growing number of genetic tests and the complexity of assessing genetic risk, there is now some movement away from completely nondirected counseling. The goal is still to provide the family with information about all options and to reach the best decision for the family, but that goal may sometimes require the counselor to recommend certain options, much as a physician recommends the most appropriate medical treatments for his or her patient.
Who does genetic counseling? In the United States, over 6000 health professionals are currently certified in genetics by the American Board of Medical Genetics or the American Board of Genetic Counseling. About half of them are specifically trained in genetic counseling, usually by completing a special 2-year masters program that provides education in both genetics and counseling. Most of the remainder are physicians and scientists certified in medical or clinical genetics. Because of the shortage of genetic counselors and medical geneticists, information about genetic testing and genetic risk is often conveyed by primary care physicians, nurses, and social workers.  TRY PROBLEM 10
TRY PROBLEM 10
152
CONCEPTS
Genetic counseling is an educational process that provides patients and their families with information about a genetic condition, its medical implications, the mode of inheritance, and reproductive options.
Genetic Testing
The ultimate goal of genetic testing is to recognize the potential for a genetic condition at an early stage. In some cases, genetic testing allows people to make informed choices about reproduction. In other cases, genetic testing allows early intervention that may lessen or even prevent the development of the condition. For those who know that they are at risk for a genetic condition, genetic testing may help alleviate anxiety associated with the uncertainty of their situation. Genetic testing includes prenatal testing and postnatal testing.
Prenatal genetic tests are those that are conducted before birth and now include procedures for diagnosing several hundred genetic diseases and disorders (Table 6.4). The major purpose of prenatal tests is to provide families with the information that they need to make choices during pregnancies and, in some cases, to prepare for the birth of a child with a genetic condition. Several approaches to prenatal diagnosis are described in the following sections.
| Disorder | Method of Detection |
|---|---|
| Chromosome abnormalities | Examination of a karyotype from cells obtained by amniocentesis or chorionic villus sampling |
| Cleft lip and palate | Ultrasound |
| Cystic fibrosis | DNA analysis of cells obtained by amniocentesis or chorionic villus sampling |
| Dwarfism | Ultrasound or X-ray; some forms can be detected by DNA analysis of cells obtained by amniocentesis or chorionic villus sampling |
| Hemophilia | Fetal blood sampling* or DNA analysis of cells obtained by amniocentesis or chorionic villus sampling |
| Lesch–Nyhan syndrome | Biochemical tests on cells obtained by amniocentesis or chorionic villus sampling |
| Neural-tube defects | Initial screening with maternal blood test, followed by biochemical tests on amniotic fluid obtained by amniocentesis or by the detection of birth defects with the use of ultrasound |
| Osteogenesis imperfecta (brittle bones) | Ultrasound or X-ray |
| Phenylketonuria | DNA analysis of cells obtained by amniocentesis or chorionic villus sampling |
| Sickle-cell anemia | Fetal blood sampling* or DNA analysis of cells obtained by amniocentesis or chorionic villus sampling |
| Tay–Sachs disease | Biochemical tests on cells obtained by amniocentesis or chorionic villus sampling |
| *A sample of fetal blood is obtained by inserting a needle into the umbilical cord. | |
Ultrasonography
Some genetic conditions can be detected through direct visualization of the fetus. This is most commonly done by ultrasonography—usually referred to as ultrasound. In this technique, high-frequency sound is beamed into the uterus; when the sound waves encounter dense tissue, they bounce back and are transformed into a picture (Figure 6.14). The size of the fetus can be determined, as can genetic conditions such as neural-tube defects (defects in the development of the spinal column and the skull) and skeletal abnormalities. Ultrasound is a standard procedure performed during pregnancy to estimate the age of the fetus, determine its sex, and check for the presence of developmental disorders or other problems.
Amniocentesis
Traditional prenatal testing requires fetal tissue, which can be obtained in several ways. The most widely used method is amniocentesis, a procedure for obtaining a sample of amniotic fluid from a pregnant woman (Figure 6.15). Amniotic fluid—the substance that fills the amniotic sac and surrounds the developing fetus—contains fetal cells that can be used for genetic testing.
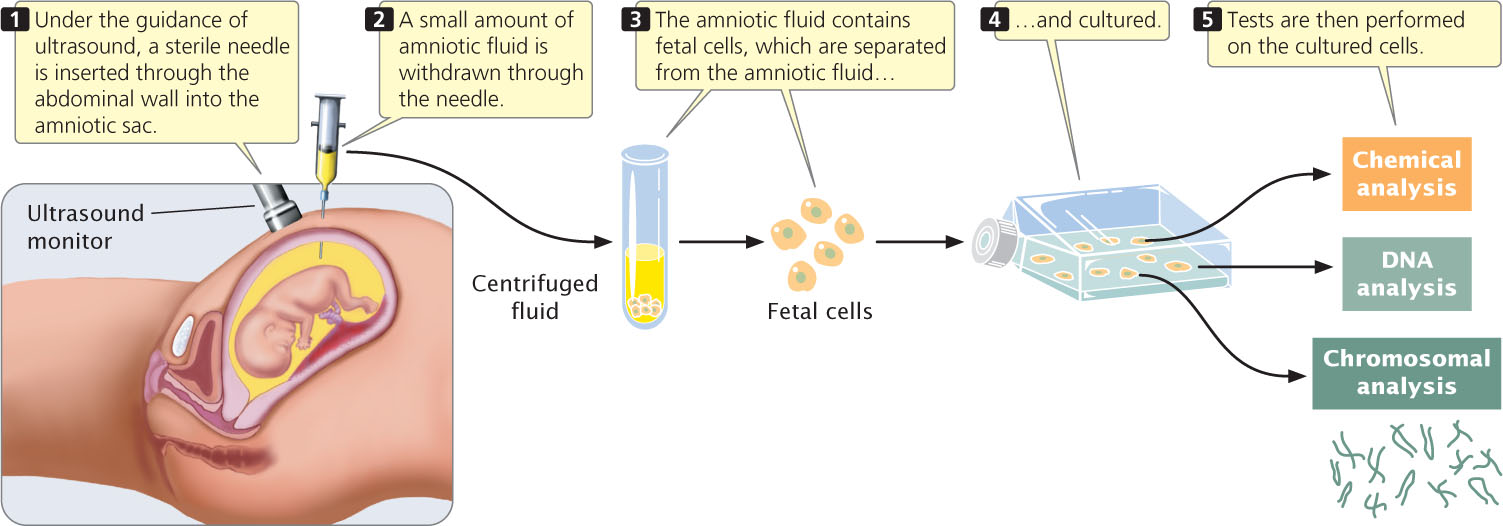
Amniocentesis is routinely performed as an outpatient procedure either with or without the use of a local anesthetic (Figure 6.15). Genetic tests are then performed on the cultured cells. Complications with amniocentesis (mostly miscarriage) are uncommon, arising in only about 1 in 400 procedures.
Chorionic Villus Sampling
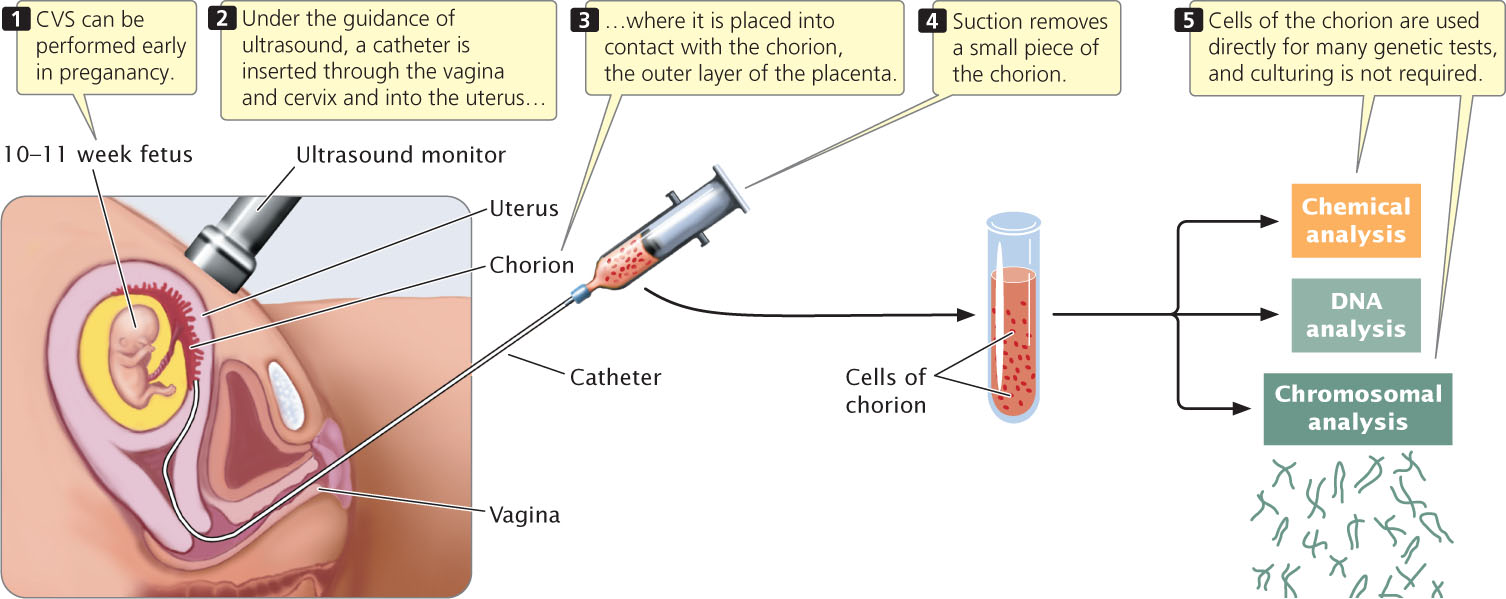
A major disadvantage of amniocentesis is that it is routinely performed at about the 15th to 18th week of a pregnancy (although some obstetricians successfully perform amniocentesis earlier). The cells obtained by amniocentesis must then be cultured before genetic tests can be performed, requiring yet more time. For these reasons, genetic information about the fetus may not be available until the 17th or 18th week of pregnancy. By this stage, abortion carries a risk of complications and is even more stressful for the parents. Chorionic villus sampling (CVS) can be performed earlier (between the 10th and 12th weeks of pregnancy) and collects a larger amount of fetal tissue, which eliminates the necessity of culturing the cells.
153
In CVS, a catheter—a soft plastic tube—is placed into contact with the chorion, the outer layer of the placenta (Figure 6.16). Suction is then applied, and a small piece of the chorion is removed. Although the chorion is composed of fetal cells, it is a part of the placenta that is expelled from the uterus after birth; the tissue that is removed is not actually from the fetus. This tissue contains millions of actively dividing cells that can be used directly in many genetic tests. Chorionic villus sampling has a somewhat higher risk of complication than that of amniocentesis; the results of several studies suggest that this procedure may increase the incidence of limb defects in the fetus when performed earlier than 10 weeks of gestation.
Fetal cells obtained by amniocentesis or by CVS can be used to prepare a karyotype, which is a picture of a complete set of metaphase chromosomes. Karyotypes can be studied for chromosome abnormalities (see Chapter 8). Biochemical analyses can be conducted on fetal cells to determine the presence of particular metabolic products of genes. For genetic diseases in which the DNA sequence of the causative gene has been determined, the DNA sequence (DNA testing; see Chapter 19) can be examined for defective alleles.
Maternal Blood Screening Tests
Increased risk of some genetic conditions can be detected by examining levels of certain substances in the blood of the mother (referred to as a maternal blood screening test). However, these tests do not determine the presence of a genetic problem; rather, they simply indicate that the fetus is at increased risk and hence are referred to as screening tests. When increased risk is detected, follow-up tests (additional blood-screening tests, ultrasound, amniocentesis, or all three) are usually conducted.
One substance examined in maternal screening tests is α-fetoprotein, a protein that is normally produced by the fetus during development and is present in fetal blood, amniotic fluid, and the mother’s blood during pregnancy. The level of α-fetoprotein is significantly higher than normal when the fetus has a neural-tube defect or one of several other disorders. Some chromosome abnormalities produce lower-than-normal levels of α-fetoprotein. Measuring the amount of α-fetoprotein in the mother’s blood gives an indication of these conditions.
154
The American College of Obstetricians and Gynecologists recommends that physicians offer all pregnant women maternal blood screening tests. One typical test, carried out between 11 and 13 weeks of pregnancy, measures human chorionic gonadotropin (hCG, a pregnancy hormone) and a substance called pregnancy-associated plasma protein A (PAPP-A). When the fetus has certain chromosomal defects, the level of PAPP-A tends to be low and the level of hCG tends to be high. The risk of a chromosomal abnormality is calculated on the basis of the levels of hCG and PAPP-A in the mother’s blood, along with the results of utrasound tests. Another test, referred to as the quad screen, measures the levels of four substances: α-fetoprotein, hCG, estriol, and inhibin. The risk of chromosomal abnormalities and certain other birth defects is calculated on the basis of the combined levels of the four substances plus the mother’s age, weight, ethnic background, and number of fetuses. The quad screen successfully detects Down syndrome (due to three copies of chromosome 21) 81% of the time.
Noninvasive Prenatal Genetic Diagnosis
Prenatal tests that utilize only maternal blood are highly desirable because they are noninvasive and pose no risk to the fetus. In addition to maternal blood screening tests, which measure chemical substances produced by the fetus or placenta, procedures called noninvasive prenatal genetic diagnosis directly examine fetal DNA in maternal blood. These tests can be performed as early as 10 weeks after conception.
During pregnancy, a few fetal cells are released into the mother’s circulatory system, where they mix and circulate with her blood. Recent advances have made it possible to detect and separate fetal cells from maternal blood cells (a procedure called fetal cell sorting) with the use of lasers and automated cell-sorting machines. The fetal cells obtained can be cultured for chromosome analysis or used as a source of fetal DNA for molecular testing (see Chapter 19). Maternal blood also contains free-floating fragments of fetal DNA, which is released from when fetal cells break down. Fetal DNA can be sequenced and tested for mutations. Tests are also available to determine the number of copies of genetic variants, allowing a determination of the number of chromosomes carried by the fetus, so that chromosome abnormalities such as Down syndrome can be detected from fetal DNA. Noninvasive prenatal genetic diagnosis is now being used to determine the blood type of the fetus, detect Down syndrome and other chromosomal disorders, and to identify mutations for genetic diseases such as cystic fibrosis and thalassemia (a blood disorder). This technology creates the potential to use a single blood sample from the mother to test for hundreds of genetic diseases and even for ordinary traits in the fetus. This possibility raises a number of social and ethical questions about the use of such information in reproductive decisions.
Preimplantation Genetic Diagnosis
Prenatal genetic tests provide today’s prospective parents with increasing amounts of information about the health of their future children. New reproductive technologies provide couples with options for using this information. One of these technologies is in vitro fertilization. In this procedure, hormones are used to induce ovulation. The ovulated eggs are surgically removed from the surface of the ovary, placed in a laboratory dish, and fertilized with sperm. The resulting embryo is then implanted in the uterus. Thousands of babies resulting from in vitro fertilization have now been born.
Genetic testing can be combined with in vitro fertilization to allow the implantation of embryos that are free of a specific genetic defect. Called preimplantation genetic diagnosis (PGD), this technique enables people who carry a genetic defect to avoid producing a child with the disorder. For example, if a woman is a carrier of an X-linked recessive disease, approximately half of her sons are expected to have the disease. Through in vitro fertilization and preimplantation testing, an embryo without the disorder can be selected for implantation in her uterus.
155
The procedure begins with the production of several single-celled embryos through in vitro fertilization. The embryos are allowed to divide several times until they reach the 8- or 16-cell stage. At this point, one cell is removed from each embryo and tested for the genetic abnormality. Removing a single cell at this early stage does not harm the embryo. After determination of which embryos are free of the disorder, a healthy embryo is selected and implanted in the woman’s uterus.
Preimplantation genetic diagnosis requires the ability to conduct a genetic test on a single cell. Such testing is possible with the use of the polymerase chain reaction through which minute quantities of DNA can be amplified (replicated) quickly (see Chapter 19). After amplification of the cell’s DNA, the DNA sequence is examined. Preimplantation genetic diagnosis has resulted in the birth of thousands of healthy children. Its use raises a number of ethical concerns, because it can be used as a means of selecting for or against genetic traits that have nothing to do with medical concerns. For example, it can potentially be used to select for a child with genes for a certain eye color or genes for increased height.
Newborn Screening
Testing for genetic disorders in newborn infants is called newborn screening. All states in the United States and many other countries require by law that newborn infants be tested for some genetic diseases and conditions. In 2006, the American College of Medical Genetics recommended mandatory screening for 29 conditions (Table 6.5), and many states have now adopted this list for newborn testing These genetic conditions were chosen because early identification can lead to effective treatment. For example, as mentioned in Chapter 5, phenylketonuria is an autosomal recessive disease that, if not treated at an early age, can result in intellectual disability. But early intervention, through the administration of a modified diet, prevents this.
| Medium-chain acyl-CoA dehydrogenase deficiency |
| Congenital hypothyroidism |
| Phenylketonuria |
| Biotinidase deficiency |
| Sickle-cell anemia (Hb SS disease) |
| Congenital adrenal hyperplasia (21-hydroxylase deficiency) |
| Isovaleric acidemia |
| Very long chain acyl-CoA dehydrogenase deficiency |
| Maple syrup (urine) disease |
| Classical galactosemia |
| Hb S β-thalassemia |
| Hb S C disease |
| Long-chain L-3-hydroxyacyl-CoA dehydrogenase deficiency |
| Glutaric acidemia type I |
| 3-Hydroxy-3-methyl glutaric aciduria |
| Trifunctional protein deficiency |
| Multiple carboxylase deficiency |
| Methylmalonic acidemia (mutase deficiency) |
| Homocystinuria (due to cystathionine β-synthase deficiency) |
| 3-Methylcrotonyl-CoA carboxylase deficiency |
| Hearing loss |
| Methylmalonic acidemia |
| Propionic acidemia |
| Carnitine uptake defect |
| β-Ketothiolase deficiency |
| Citrullinemia |
| Argininosuccinic acidemia |
| Tyrosinemia type I |
| Cystic fibrosis |
Presymptomatic Testing
In addition to testing for genetic diseases in fetuses and newborns, testing healthy adults for genes that might predispose them to a genetic condition in the future is now possible. This type of testing is known as presymptomatic genetic testing. For example, presymptomatic testing is available for members of families that have an autosomal dominant form of breast cancer. In this case, early identification of the disease-causing allele allows for closer surveillance and the early detection of tumors. Presymptomatic testing is also available for some genetic diseases for which no treatment is available, such as Huntington disease, an autosomal dominant disease that leads to slow physical and mental deterioration in middle age.
Heterozygote Screening
Another form of genetic testing in adults is heterozygote screening. In this type of screening, members of a population are tested to identify heterozygous carriers of recessive disease-causing alleles—people who are healthy but have the potential to produce children with a particular disease.
Testing for Tay–Sachs disease is a successful example of heterozygote screening. In the general population of North America, the frequency of Tay–Sachs disease is only about 1 person in 360,000. Among Ashkenazi Jews (descendants of Jewish people who settled in eastern and central Europe), the frequency is 100 times as great. A simple blood test is used to identify Ashkenazi Jews who carry the allele for Tay–Sachs disease. If a man and woman are both heterozygotes, approximately one in four of their children is expected to have Tay–Sachs disease. Couples identified as heterozygous carriers may use that information in deciding whether to have children. A prenatal test also is available for determining if the fetus of an at-risk couple will have Tay–Sachs disease. Screening programs have led to a significant decline in the number of children of Ashkenazi ancestry born with Tay–Sachs disease (now fewer than 10 children per year in the United States).  TRY PROBLEM 12
TRY PROBLEM 12
156
CONCEPTS
Genetic testing is used to screen newborns for genetic diseases, detect persons who are heterozygous for recessive diseases, detect disease-causing alleles in those who have not yet developed symptoms of the disease, and detect defective alleles in unborn babies. Preimplantation genetic diagnosis combined with in vitro fertilization allows for the selection of embryos that are free from specific genetic diseases.
 CONCEPT CHECK 9
CONCEPT CHECK 9
How does preimplantation genetic diagnosis differ from prenatal genetic testing?
Interpreting Genetic Tests
Today, more than a thousand genetic tests are clinically available and several hundred more are available through research studies. Future research will greatly increase the number and complexity of genetic tests that become available. Many of these tests will be for complex multifactorial diseases that are influenced by both genetics and environment, such as coronary artery disease, diabetes, asthma, some types of cancer, and depression.
Interpreting the results of genetic tests is often complicated by several factors. First, some genetic diseases are caused by numerous different mutations. For example, more than 1000 different mutations at a single locus can cause cystic fibrosis, an autosomal recessive disease in which chloride ion transport is defective. Genetic tests typically screen for only the most common mutations; uncommon and rare mutations are not detected. Therefore, a negative result does not mean that a genetic defect is absent; it indicates only that the person does not have a common mutation. When family members have the disease, their DNA can be examined to determine the nature of the mutation and other family members can then be screened for the same mutation, but this option is not possible if affected family members are unavailable or unwilling to be tested.
A second problem lies in interpreting the results of genetic tests. For a classic genetic disease such as Tay–Sachs disease, the inheritance of two copies of the gene virtually ensures that a person will have the disease. However, this is not the case for many genetic diseases where penetrance is incomplete and environmental factors play a role. For these conditions, carrying a disease-predisposing mutation only elevates a person’s risk of acquiring the disease. The risk associated with a particular mutation is a statistical estimate, based on the average effect of the mutation on many people. In this case, the calculated risk may provide little useful information to a specific person. It is also important to keep in mind that for many genetic traits and disorders no genetic test exists.
CONCEPTS
Interpreting genetic tests is complicated by the presence of multiple causative mutations, incomplete penetrance, and the influence of environmental factors.
Direct-to-Consumer Genetic Testing
An increasing number of genetic tests are now being offered to anyone interested in investigating his or her own hereditary conditions, without requiring a health-care provider. These direct-to-consumer genetic tests are available for testing a large and growing array of genetic conditions in adults and children, everything from single-gene disorders such as cystic fibrosis to multifactorial conditions such as obesity, cardiovascular disease, athletic performance, and predisposition to nicotine addiction. Direct-to-consumer tests are also available for paternity testing and for determining ancestry.
Many direct-to-consumer genetic tests are advertised and ordered through the Internet. After a person orders a test, the company sends a kit for collecting a sample of DNA (usually cells from saliva, the inside of the cheek, or a spot of blood). The person collects the sample and sends it back to the company, which performs the test and sends the results to the person. Geneticists, public health officials, and consumer advocates have raised a number of concerns about direct-to-consumer genetic testing, including concerns that some tests are offered without appropriate information and genetic counseling and that consumers are often not equipped to interpret the results. Other concerns focus on the accuracy of some tests, the confidentiality of the results, and whether indications of risk provided by the test are even useful.
Advocates of direct-to-consumer genetic tests contend that the tests provide greater access to testing and enhanced confidentiality. Many states do not regulate direct-to-consumer testing and, currently, there is little federal oversight.  TRY PROBLEM 15
TRY PROBLEM 15
Genetic Discrimination and Privacy
With the development of many new genetic tests, concerns have been raised about privacy regarding genetic information and the potential for genetic discrimination. Research shows that many people at risk for genetic diseases avoid genetic testing because they fear that the results would make it difficult for them to obtain health insurance or that the information might adversely affect their employability. Some of those who do seek genetic testing pay for it themselves and use aliases to prevent the results from becoming part of their health records. Fears about genetic discrimination have been reinforced by past practices. In the 1970s, some African Americans who were identified as carriers of sickle-cell anemia (an autosomal recessive disorder) faced employment discrimination and had difficulty obtaining health insurance, in spite of the fact that carriers are healthy.
157
In response to these concerns, the U.S. Congress passed the Genetic Information Nondiscrimination Act (GINA) in 2008. This law prohibits health insurers from using genetic information to make decisions about health-insurance coverage and rates. It also prevents employers from using genetic information in employment decisions and prohibits health insurers and employers from asking or requiring a person to take a genetic test. Results of genetic testing receive some degree of protection by other federal regulations that cover the uses and disclosure of individual health information. However, GINA covers health insurance and employment only; it does not apply to life, disability, and long-term care insurance.  TRY PROBLEM 16
TRY PROBLEM 16
CONCEPTS
The growing number of genetic tests and their complexity have raised several concerns, including those about direct-to-consumer tests, genetic discrimination, and privacy regarding test results.