8.2 Chromosome Rearrangements Alter Chromosome Structure
Chromosome rearrangements are mutations that change the structures of individual chromosomes. The four basic types of rearrangements are duplications, deletions, inversions, and translocations (Figure 8.4). Many of these chromosome rearrangements originate when double-stranded breaks occur in the DNA molecules found within a chromosome. Double-stranded breaks in DNA often cause cell death, so organisms have evolved elaborate mechanisms to repair breaks by reconnecting the broken ends of DNA. If the two broken ends are rejoined correctly, the original chromosome is restored and no chromosome rearrangement results. However, sometimes the wrong ends are connected, leading to a chromosome rearrangement. Chromosome rearrangements can also arise through errors in crossing over or when crossing over occurs between repeated DNA sequences.
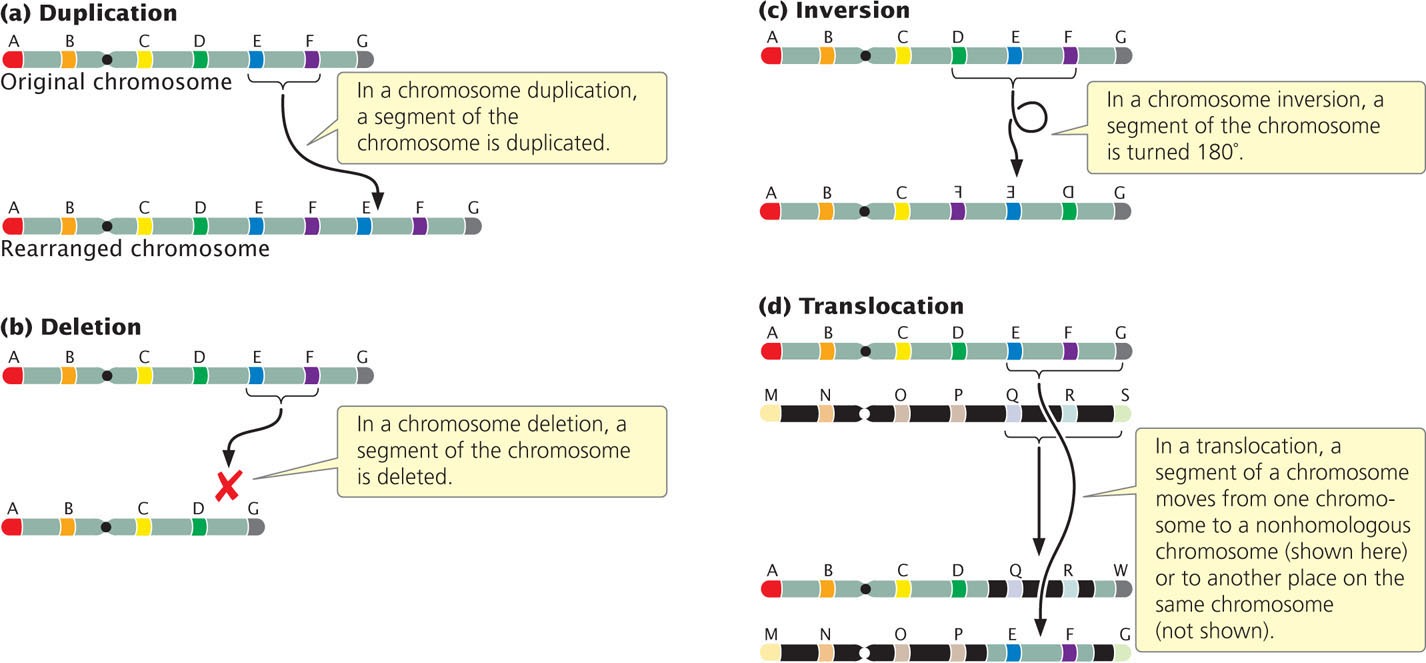
Duplications
A chromosome duplication is a mutation in which part of the chromosome has been doubled (see Figure 8.4a). Consider a chromosome with segments AB·CDEFG, in which · represents the centromere. A duplication might include the EF segments, giving rise to a chromosome with segments AB·CDEFEFG. This type of duplication, in which the duplicated region is immediately adjacent to the original segment, is called a tandem duplication. If the duplicated segment is located some distance from the original segment, either on the same chromosome or on a different one, the chromosome rearrangement is called a displaced duplication. An example of a displaced duplication would be AB·CDEFGEF. A duplication can be either in the same orientation as that of the original sequence, as in the two preceding examples, or inverted: AB·CDEFFEG. When the duplication is inverted, it is called a reverse duplication.
Effects of Chromosome Duplications
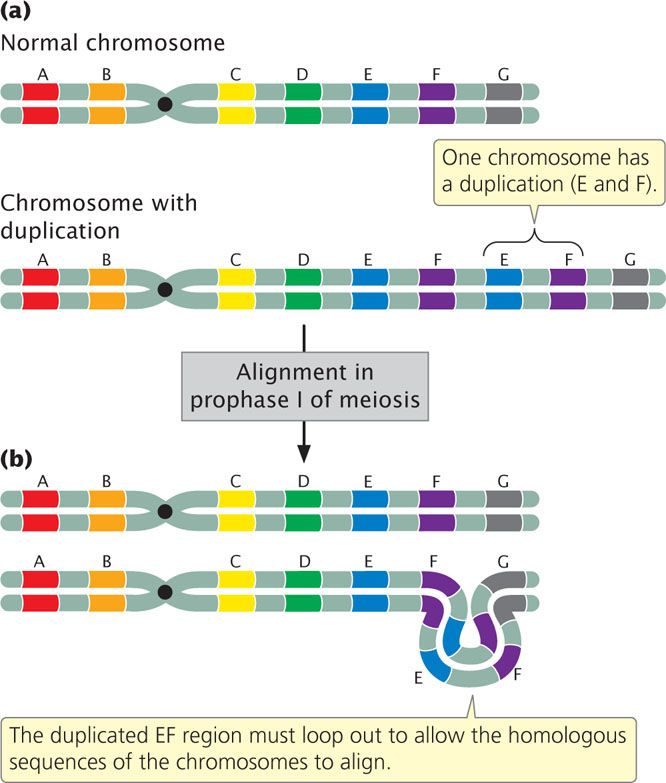
An individual homozygous for a duplication carries the duplication on both homologous chromosomes, and an individual heterozygous for a duplication has one normal chromosome and one chromosome with the duplication. In the heterozygotes (Figure 8.5a), problems arise in chromosome pairing at prophase I of meiosis because the two chromosomes are not homologous throughout their length. The pairing and synapsis of homologous regions require that one or both chromosomes loop and twist so that these regions are able to line up (Figure 8.5b). The appearance of this characteristic loop structure in meiosis is one way to detect duplications.
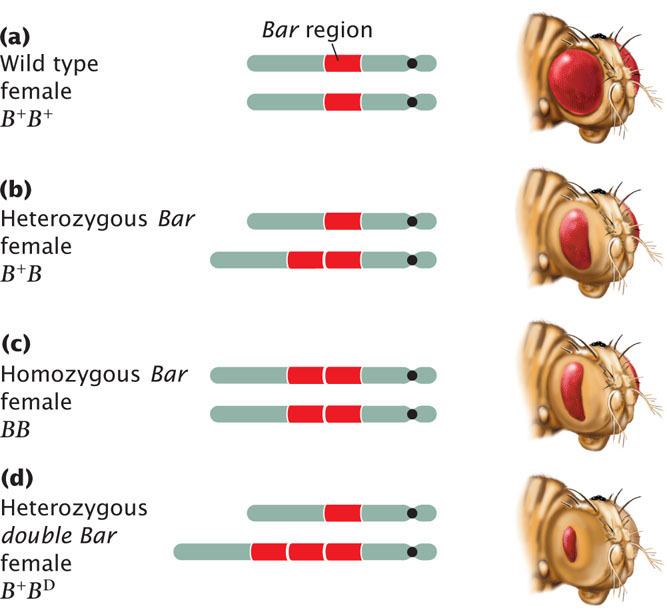
Duplications may have major effects on the phenotype. Among fruit flies, for example, a fly having a Bar mutation has a reduced number of facets in the eye, making the eye smaller and bar shaped instead of oval (Figure 8.6). The Bar mutation results from a small duplication on the X chromosome that is inherited as an incompletely dominant, X-linked trait: heterozygous female flies have somewhat smaller eyes (the number of facets is reduced; see Figure 8.6b), whereas, in homozygous female and hemizygous male flies, the number of facets is greatly reduced (see Figure 8.6c). Occasionally, a fly carries three copies of the Bar duplication on its X chromosome; for flies carrying such mutations, which are termed double Bar, the number of facets is extremely reduced (see Figure 8.6d).
213
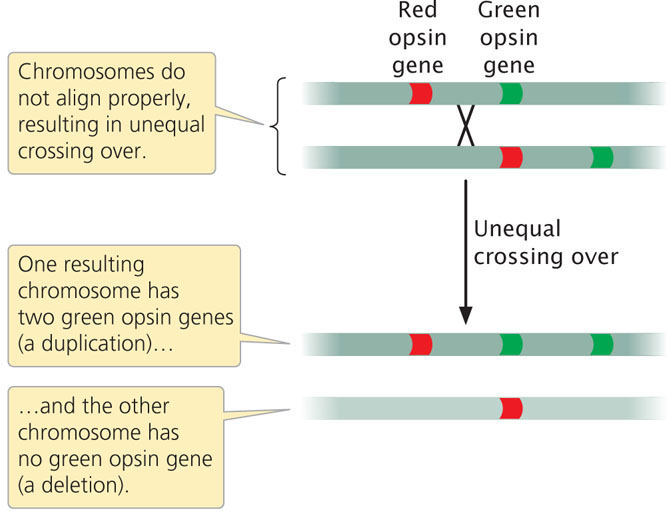
Duplications and deletions often arise from unequal crossing over, in which duplicated segments of chromosomes misalign during the process. Unequal crossing is frequently the cause of red-green color blindness in humans. Perception of color is affected by red and green opsin genes, which are found on the X chromosome and are 98% identical in their DNA sequence. Most people with normal color vision have one red opsin gene and one green opsin gene (although some people have more than one copy of each). Occasionally, two paired X chromosomes in a female do not align properly in prophase I and unequal crossing over takes place. The unequal crossing over produces one chromosome with an extra opsin gene and one chromosome that is missing an opsin gene (Figure 8.7; see also Figure 18.14). When a male inherits the chromosome that is missing one of the opsin genes, red-green color blindness results.
Unbalanced Gene Dosage
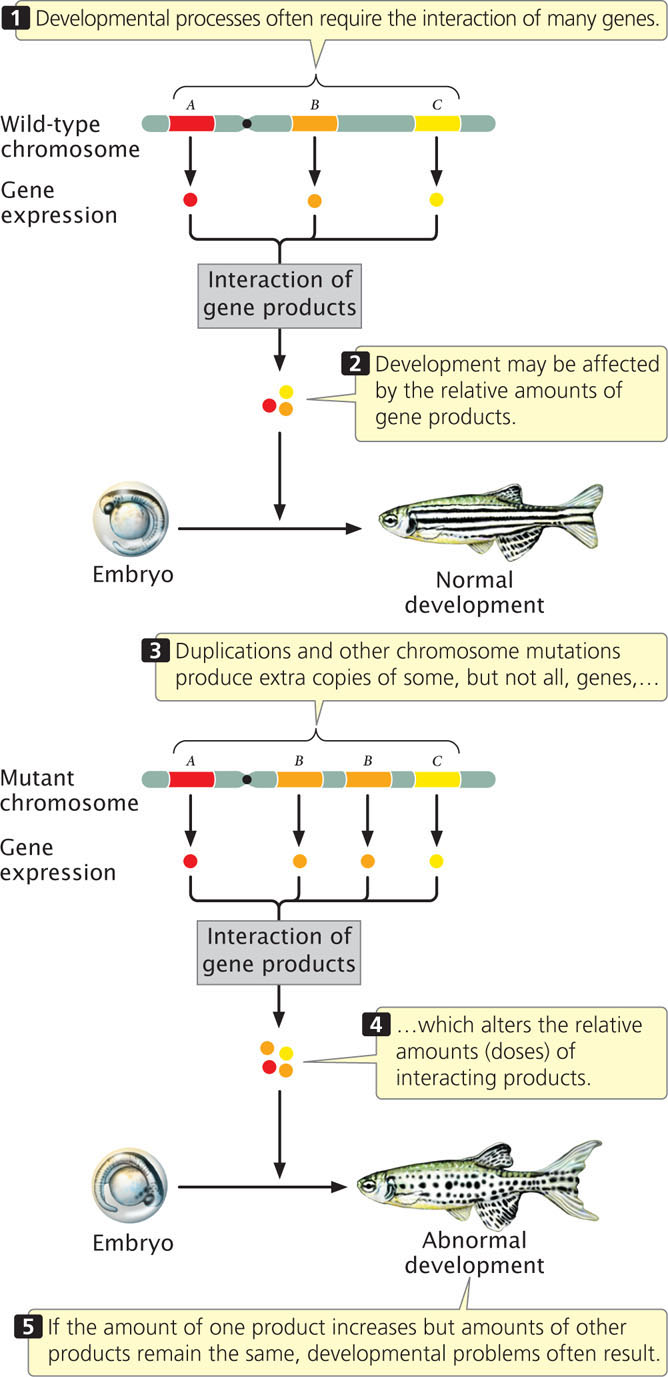
How does a chromosome duplication alter the phenotype? After all, gene sequences are not altered by duplications, and no genetic information is missing; the only change is the presence of additional copies of normal sequences. The answer to this question is not well understood, but the effects are most likely due to imbalances in the amounts of gene products (abnormal gene dosage). The amount of a particular protein synthesized by a cell is often directly related to the number of copies of its corresponding gene: an individual organism with three functional copies of a gene often produces 1.5 times as much of the protein encoded by that gene as that produced by an individual with two copies. Because developmental processes require the interaction of many proteins, they often depend critically on proper gene dosage. If the amount of one protein increases while the amounts of others remain constant, problems can result (Figure 8.8). Duplications can have severe consequences when the precise balance of a gene product is critical to cell function (Table 8.1).
| Type of Rearrangement | Chromosome | Disorder | Symptoms |
|---|---|---|---|
| Duplication | 4, short arm | — | Small head, short neck, low hairline, growth and intellectual disability |
| Duplication | 4, long arm | — | Small head, sloping forehead, hand abnormalities |
| Duplication | 7, long arm | — | Delayed development, asymmetry of the head, fuzzy scalp, small nose, low-set ears |
| Duplication | 9, short arm | — | Characteristic face, variable intellectual disability, high and broad forehead, hand abnormalities |
| Deletion | 5, short arm | Cri-du-chat syndrome | Small head, distinctive cry, widely spaced eyes, round face, intellectual disability |
| Deletion | 4, short arm | Wolf–Hirschhorn syndrome | Small head with high forehead, wide nose, cleft lip and palate, severe intellectual disability |
| Deletion | 4, long arm | — | Small head, mild to moderate intellectual disability, cleft lip and palate, hand and foot abnormalities |
| Deletion | 7, long arm | Williams–Beuren syndrome | Facial features, heart defects, mental impairment |
| Deletion | 15, long arm | Prader–Willi syndrome | Feeding difficulty at early age but becoming obese after 1 year of age, mild to moderate intellectual disability |
| Deletion | 18, short arm | — | Round face, large low-set ears, mild to moderate intellectual disability |
| Deletion | 18, long arm | — | Distinctive mouth shape, small hands, small head, intellectual disability |
214
Segmental Duplications
The human genome contains numerous duplicated sequences called segmental duplications, which are defined as duplications greater than 1000 base pairs (1000 bp) in length. In most segmental duplications, the two copies are found on the same chromosome (an intrachromosomal duplication) but, in others, the two copies are found on different chromosomes (an interchromosomal duplication). Many segmental duplications have been detected with the use of molecular techniques that examine DNA sequences on a chromosome (see Chapter 20). These techniques reveal that about 4% of the human genome consists of segmental duplications. In the human genome, the average size of segmental duplications is 15,000 bp.
Importance of Duplications in Evolution
Duplications have arisen frequently throughout the evolution of many eukaryotic organisms. Chromosome duplications provide one way in which new genes evolve. In many cases, existing copies of a gene are not free to vary, because they encode a product that is essential to development or function. However, after a chromosome undergoes duplication, extra copies of genes within the duplicated region are present. The original copy can provide the essential function, whereas an extra copy from the duplication is free to undergo mutation and change. Over evolutionary time, the extra copy may acquire enough mutations to assume a new function that benefits the organism. For example, humans have a series of genes that encode different globin chains, some of which function as an oxygen carrier during adult stages and others that function during embryonic and fetal development. All of these globin genes arose from an original ancestral gene that underwent a series of duplications.
CONCEPTS
A chromosome duplication is a mutation that doubles part of a chromosome. In individuals heterozygous for a chromosome duplication, the duplicated region of the chromosome loops out when homologous chromosomes pair in prophase I of meiosis. Duplications often have major effects on the phenotype, possibly by altering gene dosage. Segmental duplications are common within the human genome and have played an important role in the evolution of many eukaryotes.
 CONCEPT CHECK 1
CONCEPT CHECK 1
Chromosome duplications often result in abnormal phenotypes because
- developmental processes depend on the relative amounts of proteins encoded by different genes.
- extra copies of the genes within the duplicated region do not pair in meiosis.
- the chromosome is more likely to break when it loops in meiosis.
- extra DNA must be replicated, which slows down cell division.
Deletions
A second type of chromosome rearrangement is a deletion: the loss of a chromosome segment (see Figure 8.4b). A chromosome with segments AB·CDEFG that undergoes a deletion of segment EF would generate the mutated chromosome AB·CDG.
215
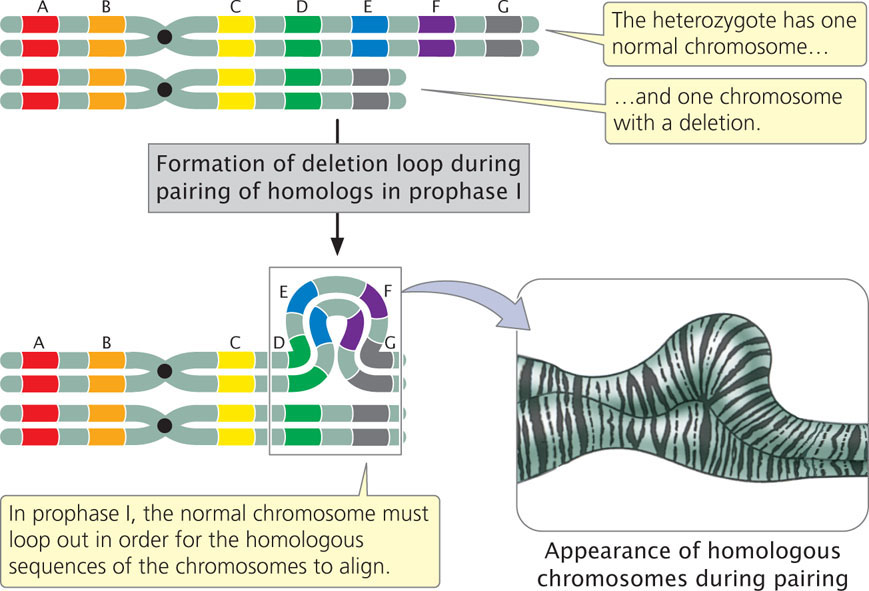
A large deletion can be easily detected because the chromosome is noticeably shortened. In individuals heterozygous for deletions, the normal chromosome must loop out during the pairing of homologs in prophase I of meiosis (Figure 8.9) to allow the homologous regions of the two chromosomes to align and undergo synapsis. This looping out generates a structure that looks very much like that seen for individuals heterozygous for duplications.
Effects of Deletions
The phenotypic consequences of a deletion depend on which genes are located in the deleted region. If the deletion includes the centromere, then the chromosome will not segregate in meiosis or mitosis and will usually be lost. Many deletions are lethal in the homozygous state because all copies of any essential genes located in the deleted region are missing. Even individuals heterozygous for a deletion may have multiple defects for three reasons.
First, the heterozygous condition may produce imbalances in the amounts of gene products, similar to the imbalances produced by extra gene copies. Second, normally recessive mutations on the homologous chromosome lacking the deletion may be expressed when the wild-type allele has been deleted (and is no longer present to mask the recessive allele’s expression). The expression of a normally recessive mutation is referred to as pseudodominance, and it is an indication that one of the homologous chromosomes has a deletion. Third, some genes must be present in two copies for normal function. When a single copy of a gene is not sufficient to produce a wild-type phenotype, it is said to be a haploinsufficient gene. A series of X-linked wing mutations in Drosophila is known as Notch. These mutations often result from chromosome deletions. Notch deletions behave as dominant mutations: when heterozygous for a Notch deletion, a fly has wings that are notched at the tips and along the edges (Figure 8.10). The Notch mutation is therefore haploinsufficient. Females that are homozygous for a Notch deletion (or males that are hemizygous) die early in embryonic development. The Notch locus, which is deleted in Notch mutations, encodes a receptor that normally transmits signals received from outside the cell to the cell’s interior and is important in fly development. The deletion acts as a recessive lethal because the loss of all copies of the Notch gene prevents normal development.

216
Chromosome Deletions in Humans
In humans, a deletion on the short arm of chromosome 5 is responsible for cri-du-chat syndrome. The name (French for “cry of the cat”) derives from the peculiar, catlike cry of infants with this syndrome. A child who is heterozygous for this deletion has a small head, widely spaced eyes, a round face, and is intellectually disabled. Deletion of part of the short arm of chromosome 4 results in another human disorder, Wolf–Hirschhorn syndrome, which is characterized by seizures, severe intellectual disability, and delayed growth. A deletion of a tiny segment of chromosome 7 causes haploinsufficiency of the gene encoding elastin and a few other genes and leads to a condition known as Williams–Beuren syndrome, which is characterized by distinctive facial features, heart defects, high blood pressure, and cognitive impairments. Effects of deletions in human chromosomes are summarized in Table 8.1.
CONCEPTS
A chromosomal deletion is a mutation in which a part of a chromosome is lost. In individuals heterozygous for a deletion, the normal chromosome loops out during prophase I of meiosis. Deletions cause recessive genes on the homologous chromosome to be expressed and may cause imbalances in gene products.
 CONCEPT CHECK 2
CONCEPT CHECK 2
What is pseudodominance and how is it produced by a chromosome deletion?
Inversions
A third type of chromosome rearrangement is a chromosome inversion, in which a chromosome segment is inverted—turned 180 degrees (see Figure 8.4c). If a chromosome originally had segments AB·CDEFG, then chromosome AB·CFEDG represents an inversion that includes segments DEF. For an inversion to take place, the chromosome must break in two places. Inversions that do not include the centromere, such as AB·CFEDG, are termed paracentric inversions (para meaning “next to”), whereas inversions that include the centromere, such as ADC·BEFG, are termed pericentric inversions (peri meaning “around”).
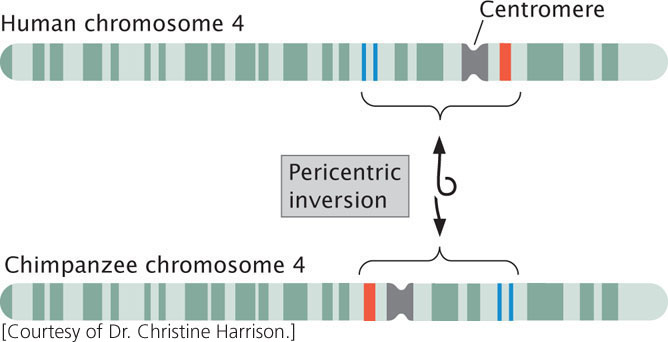
Inversion heterozygotes are common in many organisms, including a number of plants, some species of Drosophila, mosquitoes, and grasshoppers. Inversions may have played an important role in human evolution: G-banding patterns reveal that several human chromosomes differ from those of chimpanzees by only a pericentric inversion (Figure 8.11).
Effects of Inversions
Individual organisms with inversions have neither lost nor gained any genetic material; only the DNA sequence has been altered. Nevertheless, these mutations often have pronounced phenotypic effects. An inversion may break a gene into two parts, with one part moving to a new location and destroying the function of that gene. Even when the chromosome breaks are between genes, phenotypic effects may arise from the inverted gene order in an inversion. Many genes are regulated in a position-dependent manner; if their positions are altered by an inversion, their expression may be altered, an outcome referred to as a position effect. For example, when an inversion moves a wild-type allele (that normally encodes red eyes) at the white locus in Drosophila to a chromosome region that contains highly condensed and inactive chromatin, the wild-type allele is not expressed in some cells, resulting in a eye consisting of red and white spots.
217
Inversions in Meiosis
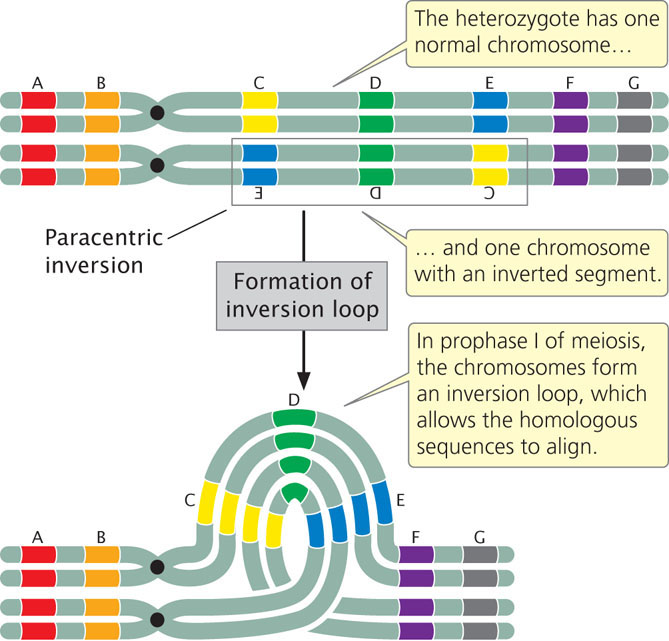
When an individual is homozygous for a particular inversion, no special problems arise in meiosis, and the two homologous chromosomes can pair and separate normally. However, when an individual is heterozygous for an inversion, the gene order of the two homologs differs, and the homologous sequences can align and pair only if the two chromosomes form an inversion loop (Figure 8.12).
Individuals heterozygous for inversions also exhibit reduced recombination among genes located in the inverted region. The frequency of crossing over within the inversion is not actually diminished but, when crossing over does take place, the outcome is abnormal gametes that result in non-viable offspring, and thus no recombinant progeny are observed. Let’s see why this happens.
Figure 8.13 illustrates the results of crossing over within a paracentric inversion: the individual is heterozygous for an inversion (see Figure 8.13a), with one wild-type, unmutated chromosome (AB·CDEFG) and one inverted chromosome (AB·EDCFG). In prophase I of meiosis, an inversion loop forms, allowing the homologous sequences to pair up (see Figure 8.13b). If a single crossover takes place in the inverted region (between segments C and D in Figure 8.13), an unusual structure results (see Figure 8.13c). The two outer chromatids, which did not participate in crossing over, contain original, nonrecombinant gene sequences. The two inner chromatids, which did participate in crossing over, are highly abnormal: each has two copies of some genes and no copies of others. Furthermore, one of the four chromatids now has two centromeres and is said to be a dicentric chromatid; the other lacks a centromere and is an acentric chromatid.
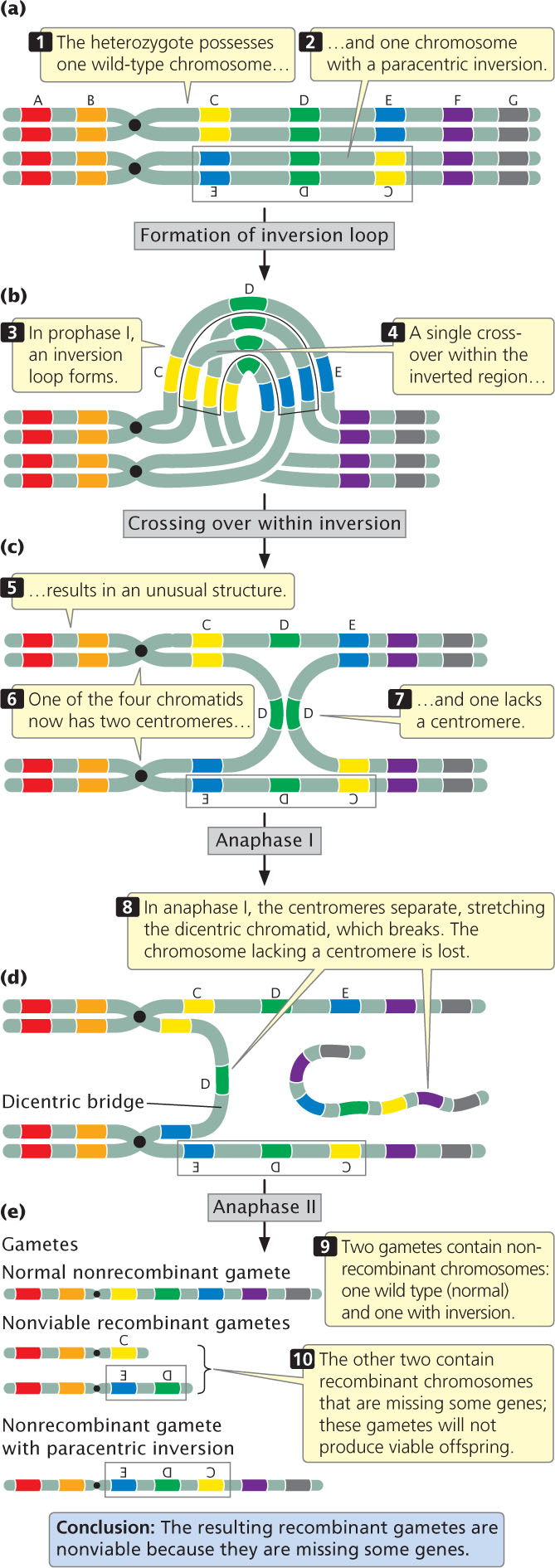
218
In anaphase I of meiosis, the centromeres are pulled toward opposite poles and the two homologous chromosomes separate. This action stretches the dicentric chromatid across the center of the nucleus, forming a structure called a dicentric bridge (see Figure 8.13d). Eventually, the dicentric bridge breaks as the two centromeres are pulled farther apart. Spindle fibers do not attach to the acentric fragment, so this fragment does not segregate to a spindle pole and is usually lost when the nucleus reforms.
In the second division of meiosis, the sister chromatids separate and four gametes are produced (see Figure 8.13e). Two of the gametes contain the original, nonrecombinant chromosomes (AB·CDEFG and AB·EDCFG). The other two gametes contain recombinant chromosomes that are missing some genes; these gametes will not produce viable offspring. Thus, no recombinant progeny result when crossing over takes place within a paracentric inversion. The key is to recognize that crossing over still takes place, but, when it does so, the resulting recombinant gametes are not viable, so no recombinant progeny are observed.
Recombination is also reduced within a pericentric inversion (Figure 8.14). No dicentric bridges or acentric fragments are produced, but the recombinant chromosomes have too many copies of some genes and no copies of others, so gametes that receive the recombinant chromosomes cannot produce viable progeny.
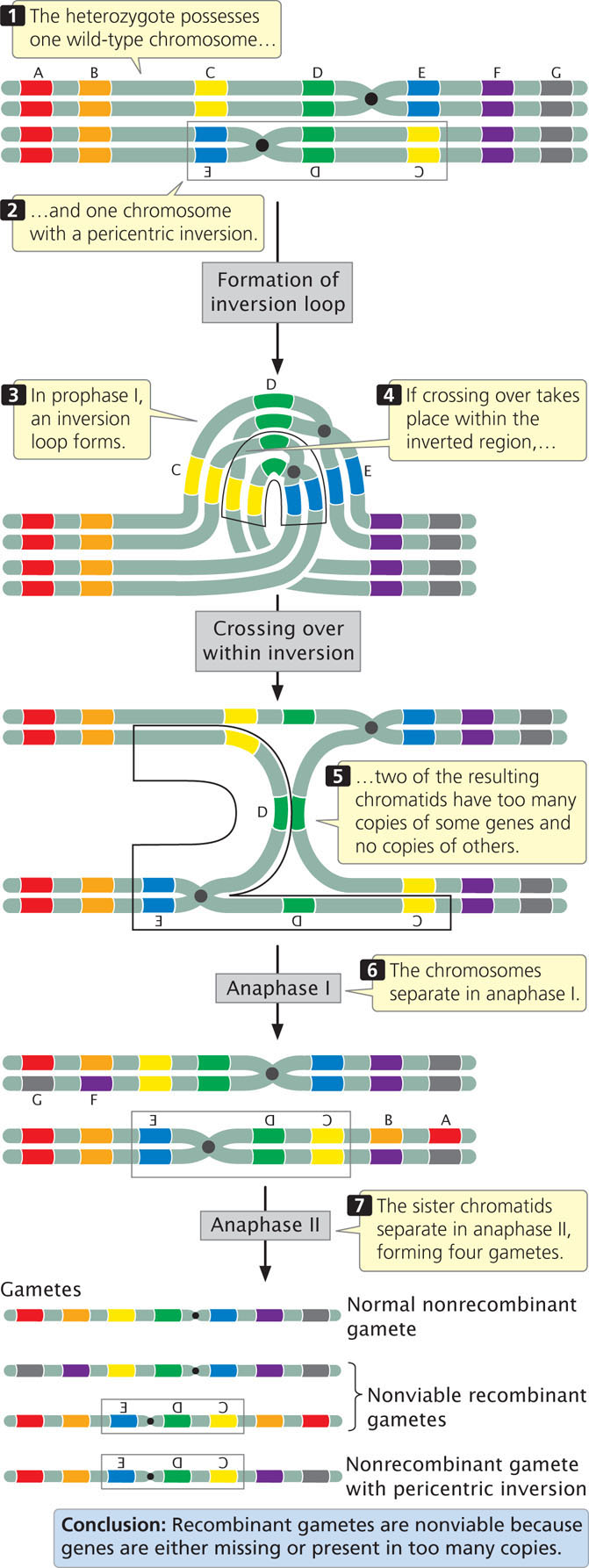
Figures 8.13 and 8.14 illustrate the results of single crossovers within inversions. Double crossovers in which both crossovers are on the same two strands (two-strand double crossovers) result in functional recombinant chromosomes (to see why functional gametes are produced by double crossovers, try drawing the results of a two-strand double crossover). Thus, even though the overall rate of recombination is reduced within an inversion, some viable recombinant progeny may still be produced through two-strand double crossovers.  TRY PROBLEM 27
TRY PROBLEM 27
Importance of Inversions in Evolution
Inversions also can play important evolutionary roles by suppressing recombination among a set of genes. As we have seen, crossing over within an inversion in an individual heterozygous for a pericentric or paracentric inversion leads to unbalanced gametes and no recombinant progeny. This suppression of recombination allows particular sets of co-adapted alleles that function well together to remain intact, unshuffled by recombination.
219
CONCEPTS
In an inversion, a segment of a chromosome is turned 180 degrees. Inversions cause breaks in some genes and may move others to new locations. In individuals heterozygous for a chromosome inversion, the homologous chromosomes form a loop in prophase I of meiosis. When crossing over takes place within the inverted region, non-viable gametes are usually produced, resulting in a depression in observed recombination frequencies.
 CONCEPT CHECK 3
CONCEPT CHECK 3
A dicentric chromosome is produced when crossing over takes place in an individual heterozygous for which type of chromosome rearrangement?
- Duplication
- Deletion
- Paracentric inversion
- Pericentric inversion
Translocations
A translocation entails the movement of genetic material between nonhomologous chromosomes (see Figure 8.4d) or within the same chromosome. Translocation should not be confused with crossing over, in which there is an exchange of genetic material between homologous chromosomes.
In a nonreciprocal translocation, genetic material moves from one chromosome to another without any reciprocal exchange. Consider the following two nonhomologous chromosomes: AB·CDEFG and MN·OPQRS. If chromosome segment EF moves from the first chromosome to the second without any transfer of segments from the second chromosome to the first, a nonreciprocal translocation has taken place, producing chromosomes AB·CDG and MN·OPEFQRS. More commonly, there is a two-way exchange of segments between the chromosomes, resulting in a reciprocal translocation. A reciprocal translocation between chromosomes AB·CDEFG and MN·OPQRS might give rise to chromosomes AB·CDQRS and MN·OPEFG.
Effects of Translocations
Translocations can affect a phenotype in several ways. First, they can physically link genes that were formerly located on different chromosomes. These new linkage relations may affect gene expression (a position effect): genes translocated to new locations may come under the control of different regulatory sequences or other genes that affect their expression.
Second, the chromosomal breaks that bring about translocations may take place within a gene and disrupt its function. Molecular geneticists have used these types of effects to map human genes. Neurofibromatosis is a genetic disease characterized by numerous fibrous tumors of the skin and nervous tissue; it results from an autosomal dominant mutation. Linkage studies first placed the locus that, when mutated, causes neurofibromatosis on chromosome 17, but the precise location of the locus was unknown. Geneticists later narrowed down the location when they identified two patients with neurofibromatosis who possessed a translocation affecting chromosome 17. These patients were assumed to have developed neurofibromatosis because one of the chromosome breaks that occurred in the translocation disrupted a particular gene, resulting in neurofibromatosis. DNA from the regions around the breaks was sequenced, eventually leading to the identification of the gene responsible for neurofibromatosis.
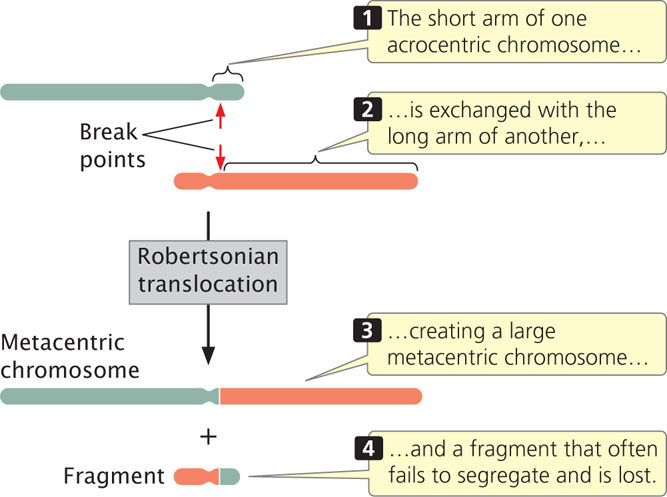
Deletions frequently accompany translocations. In a Robertsonian translocation, for example, the long arms of two acrocentric chromosomes become joined to a common centromere through a translocation, generating a metacentric chromosome with two long arms and another chromosome with two very short arms (Figure 8.15). The smaller chromosome is often lost, because very small chromosomes do not have enough mass to segregate properly during mitosis and meiosis. The result is an overall reduction in chromosome number. As we will see, Robertsonian translocations are the cause of some cases of Down syndrome, a chromosome disorder discussed later in this chapter.
Translocations in Meiosis
The effects of a translocation on chromosome segregation in meiosis depend on the nature of the translocation. Let’s consider what happens in an individual heterozygous for a reciprocal translocation. Suppose that the original chromosomes were AB·CDEFG and M·NOPQRST (designated N1 and N2 respectively, for normal chromosomes 1 and 2) and that a reciprocal translocation takes place, producing chromosomes AB·CDQRST and M·NOPEFG (designated T1 and T2, respectively, for translocated chromosomes 1 and 2). An individual heterozygous for this translocation would possess one normal copy of each chromosome and one translocated copy (Figure 8.16a). Each of these chromosomes contains segments that are homologous to two other chromosomes. When the homologous sequences pair in prophase I of meiosis, crosslike configurations consisting of all four chromosomes form (Figure 8.16b).
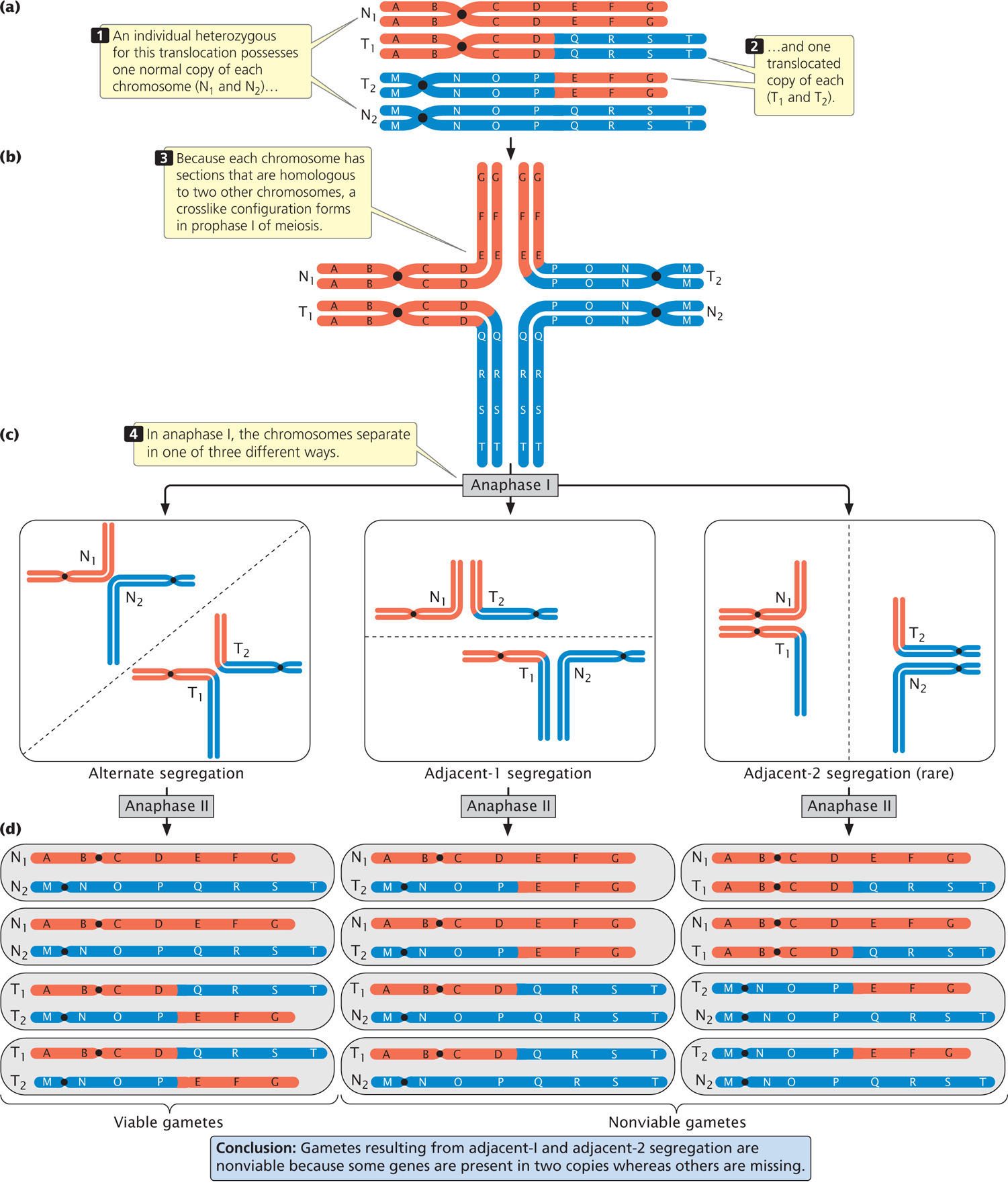
Notice that N1 and T1 have homologous centromeres (in both chromosomes, the centromere is between segments B and C); similarly, N2 and T2 have homologous centromeres (between segments N and O). Normally, homologous centromeres separate and move toward opposite poles in anaphase I of meiosis. With a reciprocal translocation, the chromosomes may segregate in three different ways. In alternate segregation (Figure 8.16c), N1 and N2 move toward one pole and T1 and T2 move toward the opposite pole. In adjacent-1 segregation, N1 and T2 move toward one pole and T1 and N2 move toward the other pole. In both alternate and adjacent-1 segregation, homologous centromeres segregate toward opposite poles. Adjacent-2 segregation, in which N1 and T1 move toward one pole and T2 and N2 move toward the other, is rare because the two homologous chromosmes usually separate in meiosis.
220
221
The products of the three segregation patterns are illustrated in Figure 8.16d. As you can see, the gametes produced by alternate segregation possess one complete set of the chromosome segments. These gametes are therefore functional and can produce viable progeny. In contrast, gametes produced by adjacent-1 and adjacent-2 segregation are not viable, because some chromosome segments are present in two copies, whereas others are missing. Because adjacent-2 segregation is rare, most gametes are produced by alternate or adjacent-1 segregation. Therefore, approximately half of the gametes from an individual heterozygous for a reciprocal translocation are expected to be functional.
The Importance of Translocations in Evolution
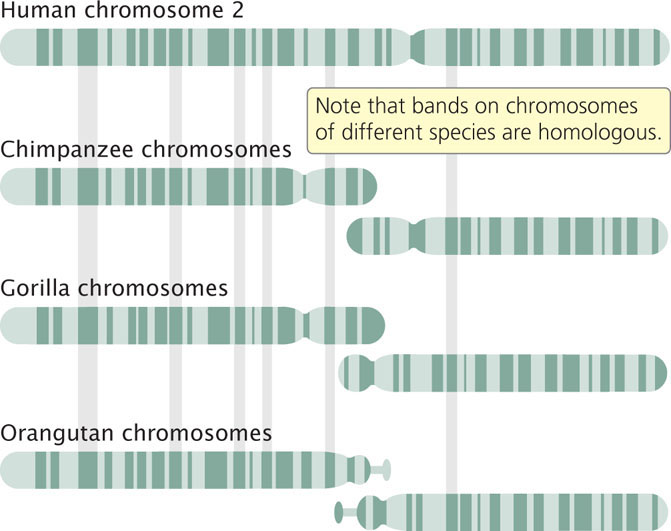
Translocations frequently play an important role in the evolution of karyotypes. Chimpanzees, gorillas, and orangutans all have 48 chromosomes, whereas humans have 46. Human chromosome 2 is a large, metacentric chromosome with G-banding patterns that match those found on two different acrocentric chromosomes of the apes (Figure 8.17). Apparently, a Robertsonian translocation took place in a human ancestor, creating a large metacentric chromosome from the two long arms of the ancestral acrocentric chromosomes, and a small chromosome consisting of the two short arms. The small chromosome was subsequently lost, leading to the reduced chromosome number in humans relative to that of the other apes.  TRY PROBLEM 28
TRY PROBLEM 28
CONCEPTS
In translocations, parts of chromosomes move to other nonhomologous chromosomes or to other regions of the same chromosome. Translocations can affect the phenotype by causing genes to move to new locations, where they come under the influence of new regulatory sequences, or by breaking genes and disrupting their function.
 CONCEPT CHECK 4
CONCEPT CHECK 4
What is the outcome of a Robertsonian translocation?
- Two acrocentric chromosomes
- One large metacentric chromosome and one very small chromosome with two very short arms
- One large metacentric and one large acrocentric chromosome
- Two large metacentric chromosomes
Fragile Sites
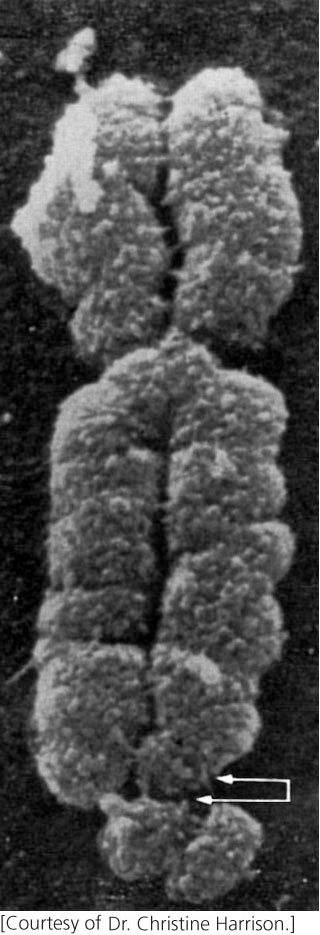
Chromosomes of cells grown in culture sometimes develop constrictions or gaps at particular locations called fragile sites (Figure 8.18) because they are prone to breakage under certain conditions. More than 100 fragile sites have been identified on human chromosomes.
Fragile sites fall into two groups. Common fragile sites are present in all humans and are a normal feature of chromosomes. Common fragile sites are often the location of chromosome breakage and rearrangements in cancer cells, leading to chromosome deletions, translocations, and other chromosome rearrangements. Rare fragile sites are found in few people and are inherited as a Mendelian trait. Rare fragile sites are often associated with genetic disorders, such as intellectual disability. Most of them consist of expanding nucleotide repeats, in which the number of repeats of a set of nucleotides is increased (see Chapter 18).
One of the most intensively studied rare fragile sites is located on the human X chromosome and is associated with fragile-X syndrome, a disorder that includes intellectual disability. Exhibiting X-linked inheritance and arising with a frequency of about 1 in 5000 male births, fragile-X syndrome has been shown to result from an increase in the number of repeats of a CGG trinucleotide.
222
Molecular studies of fragile sites have shown that many of these sites are more than 100,000 bp in length and include one or more genes. Fragile sites are often late in being replicated. At these places, the enzymes that replicate DNA may stall while unwinding of the DNA continues (see Chapter 12), leading to long stretches of DNA that are unwound and vulnerable to breakage. In spite of recent advances in our understanding of fragile sites, their nature is not completely understood.
Copy-Number Variations
Chromosome rearrangements have traditionally been detected by examination of the chromosomes with a microscope. Visual examination identifies chromosome rearrangements on the basis of changes in the overall size of a chromosome, alteration of banding patterns revealed by chromosome staining, or the behavior of chromosomes in meiosis. Microscopy, however, can detect only large chromosome rearrangements, typically those that are at least 5 million base pairs in length.
With the completion of the Human Genome Project (see Chapter 20), detailed information about DNA sequences found on individual chromosomes became available. Using this information, geneticists can now examine the number of copies of specific DNA sequences present in a cell and detect duplications, deletions, and other chromosome rearrangements that cannot be observed with microscopy alone. The work has been greatly facilitated by the availability of microarrays (see Chapter 20), which allow the simultaneous detection of hundreds of thousands of specific DNA sequences across the genome. Because these methods measure the number of copies of particular DNA sequences, the variations that they detect are called copy-number variations (CNVs). Copy-number variations include duplications and deletions that range in length from thousands of base pairs to several million base pairs. Many of these variants encompass at least one gene and may encompass several genes.
Recent studies of copy-number variation have revealed that submicroscopic chromosome duplications and deletions are quite common: research suggests that each person may possess as many as 1000 copy-number variations. Many probably have no observable phenotypic effects, but some copy-number variations have now been implicated in causing a number of diseases and disorders. For example, Janine Wagenstaller and her colleagues studied copy-number variation in 67 children with unexplained intellectual disability and found that 11 (16%) of them had duplications or deletions. Copy-number variations have also been associated with osteoporosis, autism, schizophrenia, and a number of other diseases and disorders.  TRY PROBLEM 20
TRY PROBLEM 20
CONCEPTS
Variations in the number of copies of particular DNA sequences (copy-number variations) are surprisingly common in the human genome.