9.2 Bacteria Exchange Genes Through Conjugation, Transformation, and Transduction
Bacteria exchange genetic material by three different mechanisms, all entailing some type of DNA transfer and recombination between the transferred DNA and the bacterial chromosome.
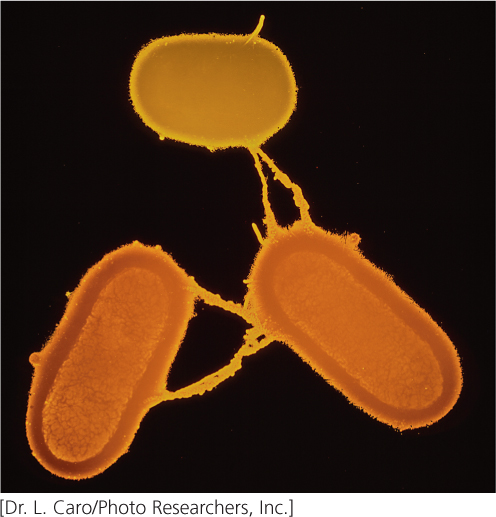
- 1. Conjugation takes place when genetic material passes directly from one bacterium to another (Figure 9.7a). In conjugation, two bacteria lie close together and a connection forms between them. A plasmid or a part of the bacterial chromosome passes from one cell (the donor) to the other (the recipient). Subsequent to conjugation, crossing over may take place between homologous sequences in the transferred DNA and the chromosome of the recipient cell. In conjugation, DNA is transferred only from donor to recipient, with no reciprocal exchange of genetic material.
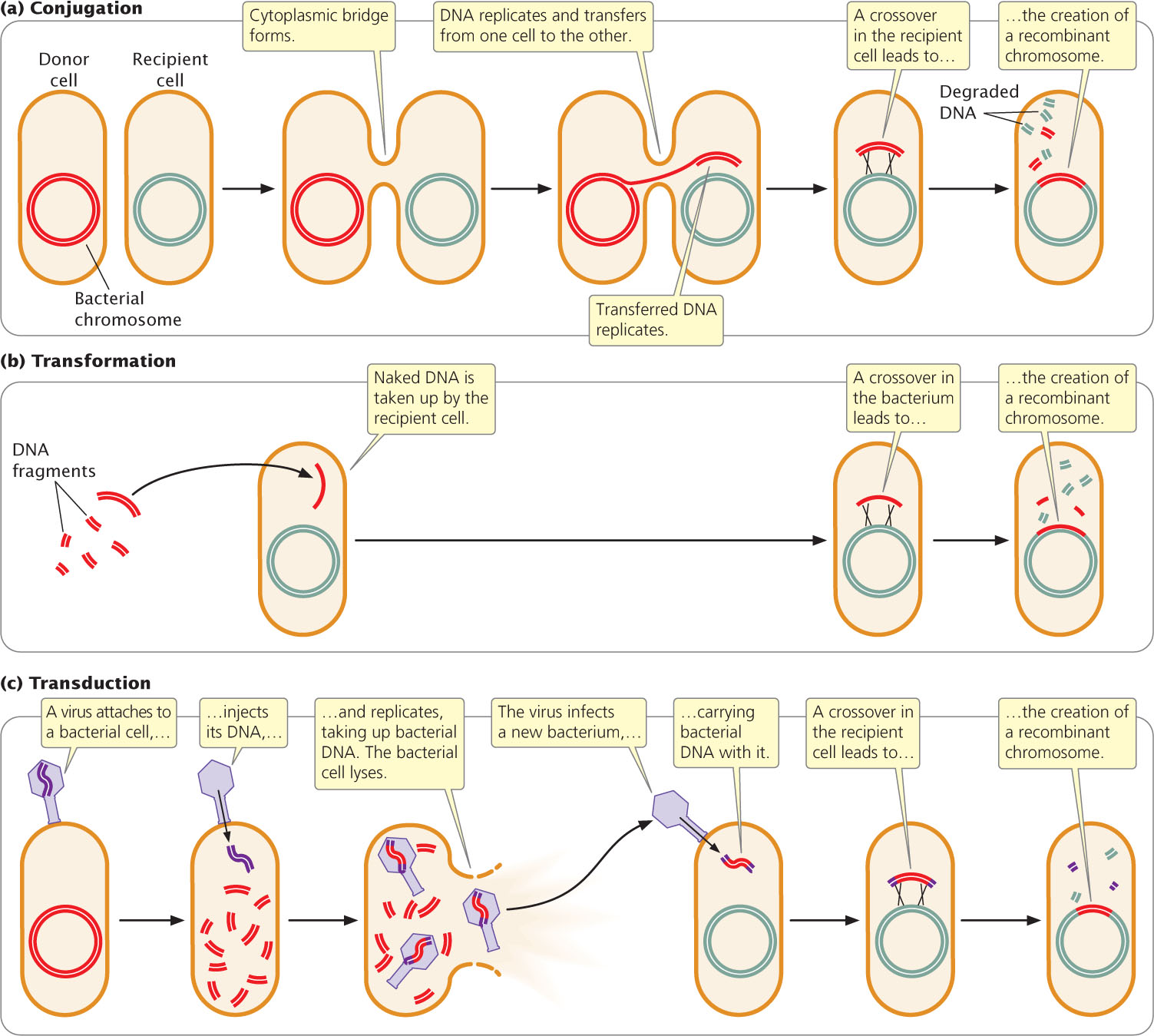 Figure 9.7: Conjugation, transformation, and transduction are three processes of gene transfer in bacteria. For the transferred DNA to be stably inherited, all three processes require the transferred DNA to undergo recombination with the bacterial chromosome.
Figure 9.7: Conjugation, transformation, and transduction are three processes of gene transfer in bacteria. For the transferred DNA to be stably inherited, all three processes require the transferred DNA to undergo recombination with the bacterial chromosome.
- 2. Transformation takes place when a bacterium takes up DNA from the medium in which it is growing (Figure 9.7b). After transformation, recombination may take place between the introduced genes and those of the bacterial chromosome.
- 3. Transduction takes place when bacterial viruses (bacteriophages) carry DNA from one bacterium to another (Figure 9.7c). Inside the bacterium, the newly introduced DNA may undergo recombination with the bacterial chromosome.
Not all bacterial species exhibit all three types of genetic transfer. Conjugation takes place more frequently in some species than in others. Transformation takes place to a limited extent in many species of bacteria, but laboratory techniques increase the rate of DNA uptake. Most bacteriophages have a limited host range; so transduction is normally between bacteria of the same or closely related species only.
These processes of genetic exchange in bacteria differ from diploid eukaryotic sexual reproduction in two important ways. First, DNA exchange and reproduction are not coupled in bacteria; bacteria often undergo reproduction (cell division) without receiving any DNA from another cell. Second, donated genetic material that is not recombined into the host DNA is usually degraded, and so the recipient cell remains haploid. Each type of genetic transfer can be used to map genes, as will be discussed in the following sections.
CONCEPTS
DNA may be transferred between bacterial cells through conjugation, transformation, or transduction. Each type of genetic transfer consists of a one-way movement of genetic information to the recipient cell, sometimes followed by recombination. These processes are not connected to cellular reproduction in bacteria.
 CONCEPT CHECK 2
CONCEPT CHECK 2
Which process of DNA transfer in bacteria requires a virus?
- Conjugation
- Transduction
- Transformation
- All of the above
Conjugation
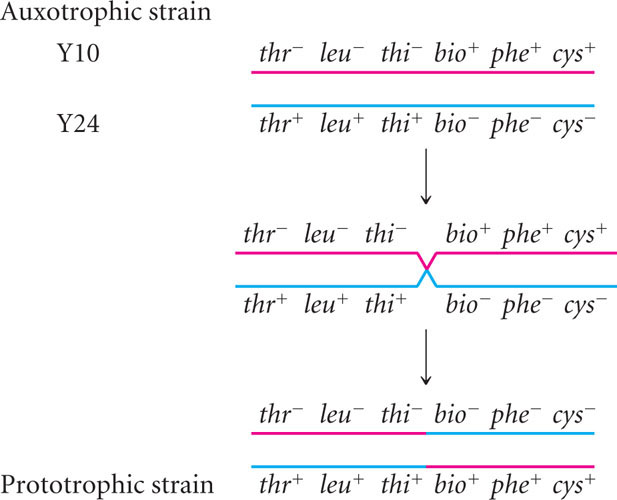
In 1946, Joshua Lederberg and Edward Tatum demonstrated that bacteria can transfer and recombine genetic information, paving the way for the use of bacteria in genetic studies. In the course of their research, Lederberg and Tatum studied auxotrophic strains of E. coli. The Y10 strain required the amino acids threonine (and was genotypically thr−) and leucine (leu−) and the vitamin thiamine (thi−) for growth but did not require the vitamin biotin (bio+) or the amino acids phenylalanine (phe+) and cysteine (cys+); the genotype of this strain can be written as thr− leu− thi− bio+ phe+ cys+. The Y24 strain had the opposite set of alleles: it required biotin, phenylalanine, and cysteine in its medium, but it did not require threonine, leucine, or thiamine; its genotype was thr+ leu+ thi+ bio− phe− cys−. In one experiment, Lederberg and Tatum mixed Y10 and Y24 bacteria together and plated them on minimal medium (Figure 9.8). Each strain was also plated separately on minimal medium.
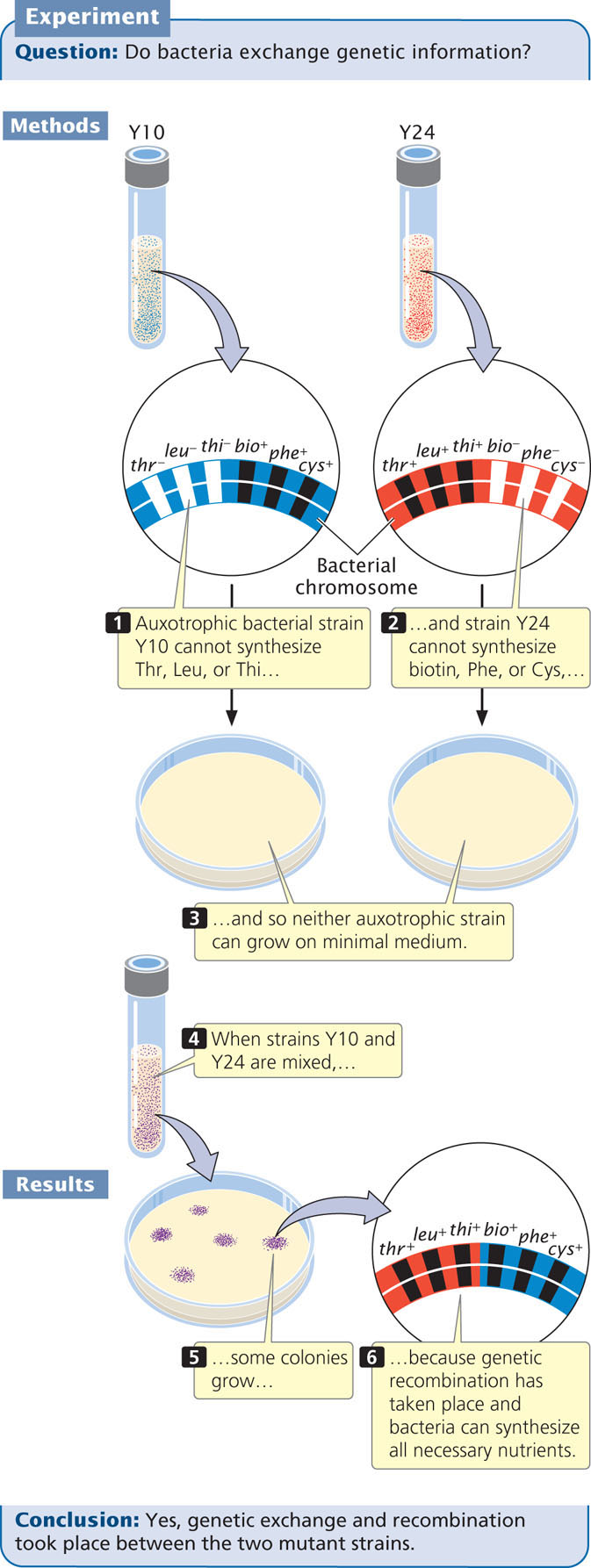
Alone, neither Y10 nor Y24 grew on minimal medium: each strain required nutrients that were absent. Strain Y10 was unable to grow, because it required threonine, leucine, and thiamine, which were absent in the minimal medium; strain Y24 was unable to grow, because it required biotin, phenylalanine, and cysteine, which also were absent from the minimal medium. When Lederberg and Tatum mixed the two strains, however, a few colonies did grow on the minimal medium. These prototrophic bacteria must have had genotype thr+ leu+ thi+ bio+ phe+ cys+. Where had they come from?
If mutations were responsible for the prototrophic colonies, then some colonies should also have grown on the plates containing Y10 or Y24 alone, but no bacteria grew on these plates. Multiple simultaneous mutations (thr− → thr+, leu− → leu+, and thi− → thi+ in strain Y10 or bio− → bio+, phe− → phe+, and cys− → cys+ in strain Y24) would have been required for either strain to become prototrophic by mutation, which was very improbable. Lederberg and Tatum concluded that some type of genetic transfer and recombination had taken place:
248
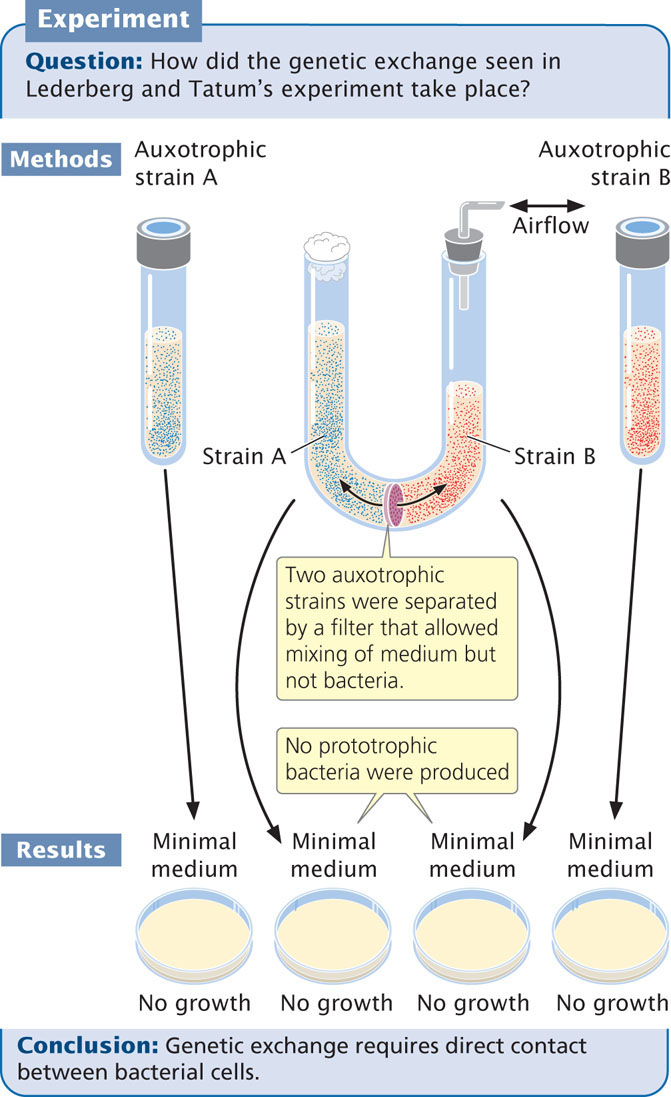
What they did not know was how it had taken place.
To study this problem, Bernard Davis constructed a U-shaped tube (Figure 9.9) that was divided into two compartments by a filter with fine pores. This filter allowed liquid medium to pass from one side of the tube to the other, but the pores of the filter were too small to allow the passage of bacteria. Two auxotrophic strains of bacteria were placed on opposite sides of the filter, and suction was applied alternately to the ends of the U-tube, causing the medium to flow back and forth between the two compartments. Despite hours of incubation in the U-tube, bacteria plated out on minimal medium did not grow; there had been no genetic exchange between the strains. The exchange of bacterial genes clearly required direct contact, or conjugation, between the bacterial cells.
F+ and F− cells
In most bacteria, conjugation depends on a fertility (F) factor that is present in the donor cell and absent in the recipient cell. Cells that contain F are referred to as F+, and cells lacking F are F−.
The F factor contains an origin of replication and a number of genes required for conjugation (see Figure 9.6). For example, some of these genes encode sex pili (singular, pilus), slender extensions of the cell membrane. A cell containing F produces the sex pili, one of which makes contact with a receptor on an F− cell (Figure 9.10) and pulls the two cells together. DNA is then transferred from the F+ cell to the F− cell. Conjugation can take place only between a cell that possesses F and a cell that lacks F.
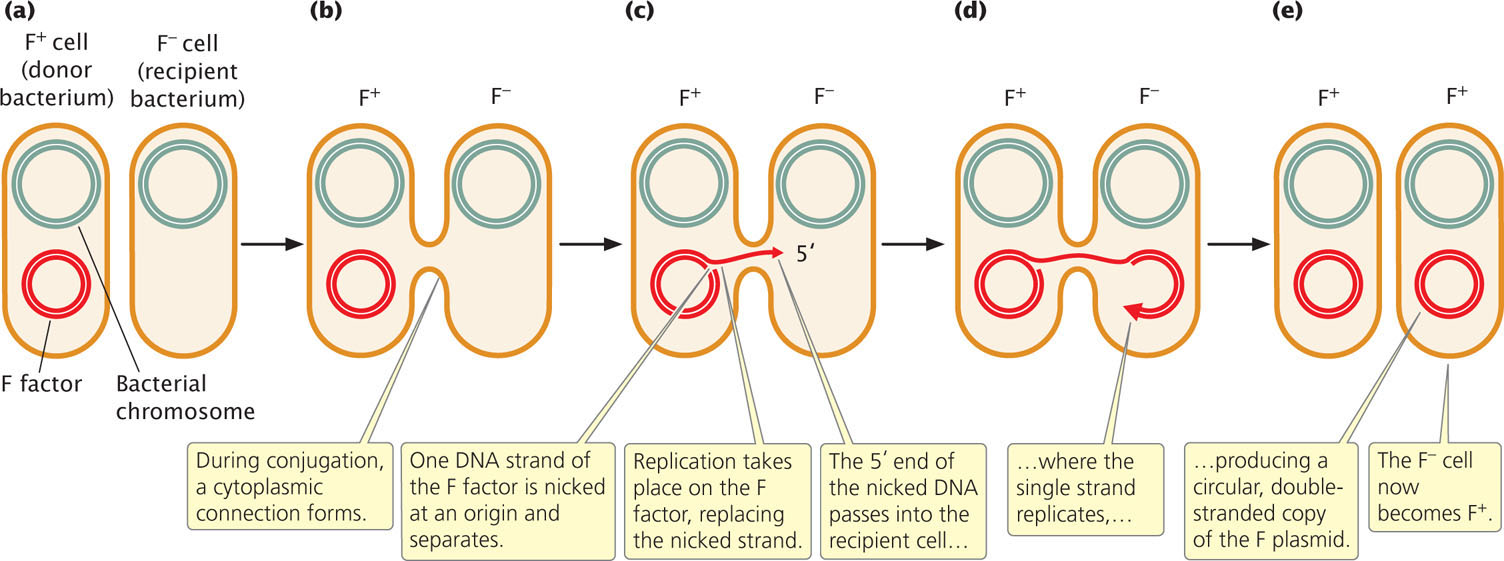
In most cases, the only genes transferred during conjugation between an F+ and F− cell are those on the F factor (Figure 9.11a and b). Transfer is initiated when one of the DNA strands on the F factor is nicked at an origin (oriT). One end of the nicked DNA separates from the circle and passes into the recipient cell (Figure 9.11c). Replication takes place on the nicked strand, proceeding around the circular plasmid in the F+ cell and replacing the transferred strand (Figure 9.11d). Because the plasmid in the F+ cell is always nicked at the oriT site, this site always enters the recipient cell first, followed by the rest of the plasmid. Thus, the transfer of genetic material has a defined direction. Inside the recipient cell, the single strand replicates, producing a circular, double-stranded copy of the F plasmid (Figure 9.11e). If the entire F factor is transferred to the recipient F− cell, that cell becomes an F+ cell.
249
Hfr Cells
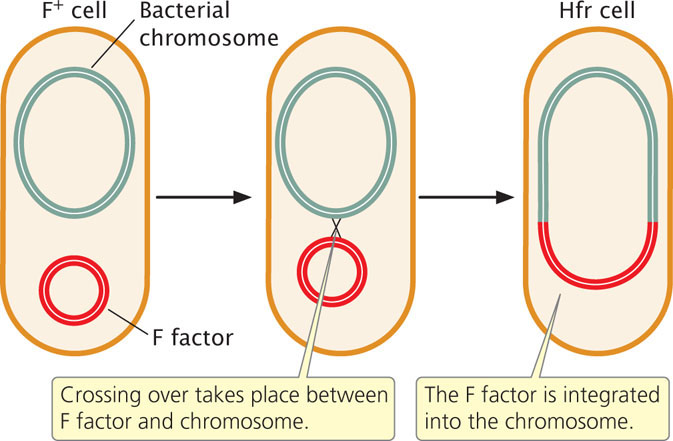
Conjugation transfers genetic material in the F plasmid from F+ to F− cells but does not account for the transfer of chromosomal genes observed by Lederberg and Tatum. In Hfr (high-frequency) strains, the F factor is integrated into the bacterial chromosome (Figure 9.12). Hfr cells behave as F+ cells, forming sex pili and undergoing conjugation with F− cells.
250
In conjugation between Hfr and F− cells (Figure 9.13a), the integrated F factor is nicked, and the end of the nicked strand moves into the F− cell (Figure 9.13b), just as it does in conjugation between F+ and F− cells. Because, in an Hfr cell, the F factor has been integrated into the bacterial chromosome, the chromosome follows it into the recipient cell. How much of the bacterial chromosome is transferred depends on the length of time that the two cells remain in conjugation.
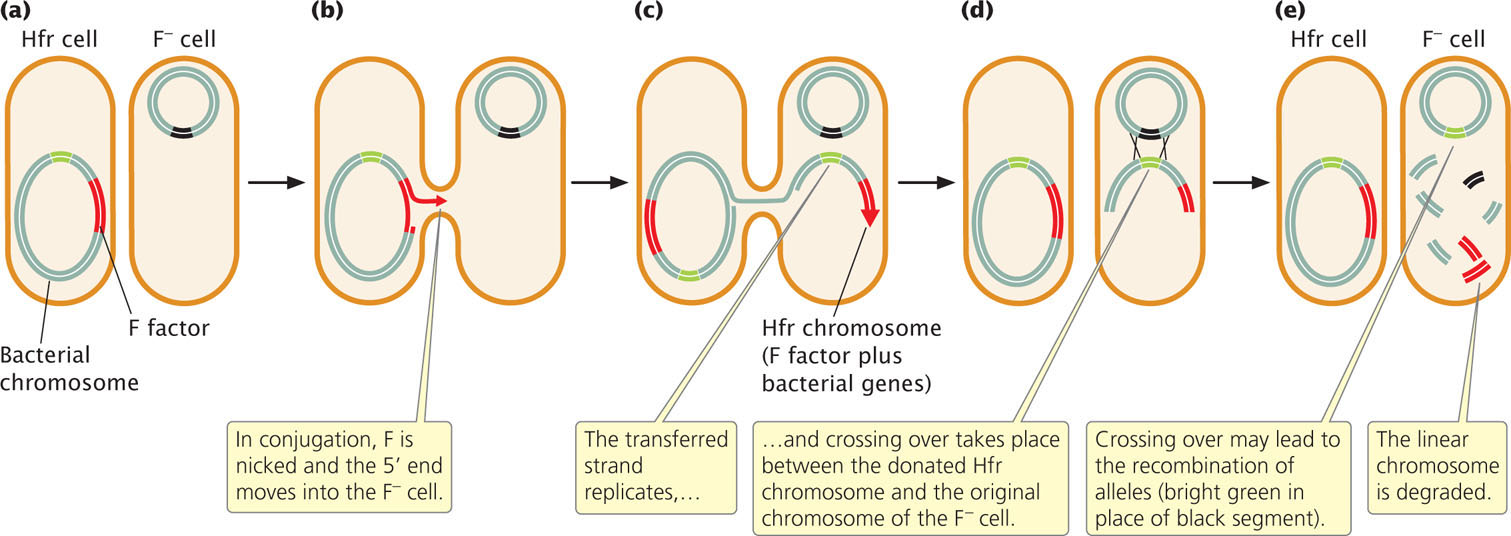
Inside the recipient cell, the donor DNA strand replicates (Figure 9.13c), and crossing over between it and the original chromosome of the F− cell (Figure 9.13d) may take place. This gene transfer between Hfr and F− cells is how the recombinant prototrophic cells observed by Lederberg and Tatum were produced. After crossing over has taken place in the recipient cell, the donated chromosome is degraded and the recombinant recipient chromosome remains (Figure 9.13e), to be replicated and passed on to later generations by binary fission.
In a mating of Hfr × F−, the F− cell almost never becomes F+ or Hfr because the F factor is nicked in the middle in the initiation of strand transfer, placing part of F at the beginning and part at the end of the strand to be transferred. To become F+ or Hfr, the recipient cell must receive the entire F factor, requiring the entire bacterial chromosome to be transferred. This event happens rarely, because most conjugating cells break apart before the entire chromosome has been transferred.
The F plasmid in F+ cells integrates into the bacterial chromosome, causing an F+ cell to become Hfr, at a frequency of only about 1 in 10,000. This low frequency accounts for the low rate of recombination observed by Lederberg and Tatum in their F+ cells. The F factor is excised from the bacterial chromosome at a similarly low rate, causing a few Hfr cells to become F+.
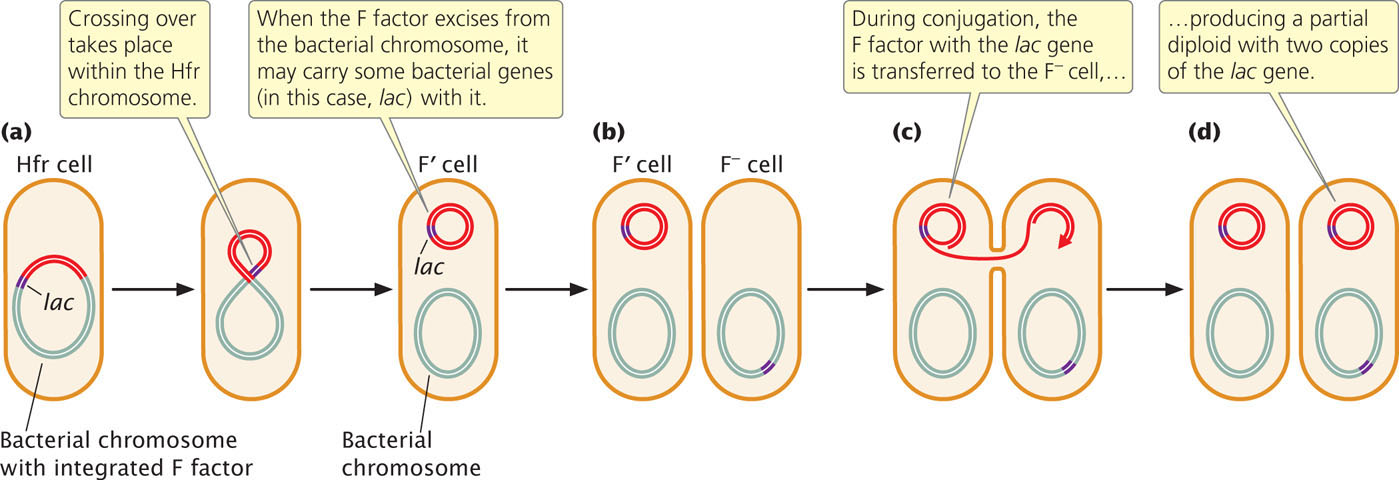
F′ Cells
When an F factor does excise from the bacterial chromosome, a small amount of the bacterial chromosome may be removed with it, and these chromosomal genes will then be carried with the F plasmid (Figure 9.14). Cells containing an F plasmid with some bacterial genes are called F prime (F′). For example, if an F factor integrates into a chromosome adjacent to the lac genes (genes that enable a cell to metabolize the sugar lactose), the F factor may pick up lac genes when it excises, becoming F′ lac. F′ cells can conjugate with F− cells because F′ cells possess the F plasmid with all the genetic information necessary for conjugation and gene transfer. Characteristics of different mating types of E. coli (cells with different types of F) are summarized in Table 9.2.
| Type | F Factor Characteristics | Role in Conjugation |
|---|---|---|
| F+ | Present as separate circular DNA | Donor |
| F− | Absent | Recipient |
| Hfr | Present, integrated into bacterial chromosome | High-frequency donor |
| F′ | Present as separate circular DNA, carrying some bacterial genes | Donor |
251
During conjugation between an F′ lac cell and an F− cell, the F plasmid is transferred to the F− cell, which means that any genes on the F plasmid, including those from the bacterial chromosome, may be transferred to F− recipient cells. This process is called sexduction. It produces partial diploids, or merozygotes, which are cells with two copies of some genes, one on the bacterial chromosome and one on the newly introduced F plasmid. The outcomes of conjugation between different mating types of E. coli are summarized in Table 9.3.
| Conjugating | Cell Types Present after Conjugation |
|---|---|
| F+ × F− | Two F+ cells (F− cell becomes F+) |
| Hfr × F− | One Hfr cell and one F− (no change)* |
| F′ × F− | Two F′ cells (F− cell becomes F′) |
| *Rarely, the F− cell becomes F+ in an Hfr × F− conjugation if the entire chromosome is transferred during conjugation. | |
CONCEPTS
Conjugation in E. coli is controlled by an episome called the F factor. Cells containing F (F+ cells) are donors during gene transfer; cells lacking F (F− cells) are recipients. Hfr cells possess F integrated into the bacterial chromosome; they donate DNA to F− cells at a high frequency. F′ cells contain a copy of F with some bacterial genes.
 CONCEPT CHECK 3
CONCEPT CHECK 3
Conjugation between an F+ and an F− cell usually results in
- two F+ cells.
- two F− cells.
- an F+ and an F− cell.
- an Hfr cell and an F+ cell.
Mapping Bacterial Genes with Interrupted Conjugation
The transfer of DNA that takes place during conjugation between Hfr and F− cells allows bacterial genes to be mapped. In conjugation, the chromosome of the Hfr cell is transferred to the F− cell. Transfer of the entire E. coli chromosome requires about 100 minutes; if conjugation is interrupted before 100 minutes have elapsed, only part of the chromosome will have passed into the F− cell and have had an opportunity to recombine with the recipient chromosome.
Chromosome transfer always begins within the integrated F factor and proceeds in a continuous direction so genes are transferred according to their sequence on the chromosome. The time required for individual genes to be transferred indicates their relative positions on the chromosome. In most genetic maps, distances are expressed as percent recombination; however, in bacterial maps constructed with interrupted conjugation, the basic unit of distance is a minute. View  Animation 9.1 to see how genes are mapped using interrupted conjugation.
Animation 9.1 to see how genes are mapped using interrupted conjugation.
252
WORKED PROBLEM
To illustrate the method of mapping genes with interrupted conjugation, let’s look at a cross analyzed by François Jacob and Elie Wollman, who developed this method of gene mapping (Figure 9.15a). They used donor Hfr cells that were sensitive to the antibiotic streptomycin (genotype strs), resistant to sodium azide (azir) and infection by bacteriophage T1 (ton1), prototrophic for threonine (thr+) and leucine (leu+), and able to break down lactose (lac+) and galactose (gal+). They used F− recipient cells that were resistant to streptomycin (strr), sensitive to sodium azide (azis) and to infection by bacteriophage T1 (tons), auxotrophic for threonine (thr−) and leucine (leu−), and unable to break down lactose (lac−) and galactose (gal−). Thus, the genotypes of the donor and recipient cells were:
Donor Hfr cells: strs leu+ thr+ azir tonr lac+ gal+
Recipient F− cells: strr leu− thr− azis tons lac− gal−
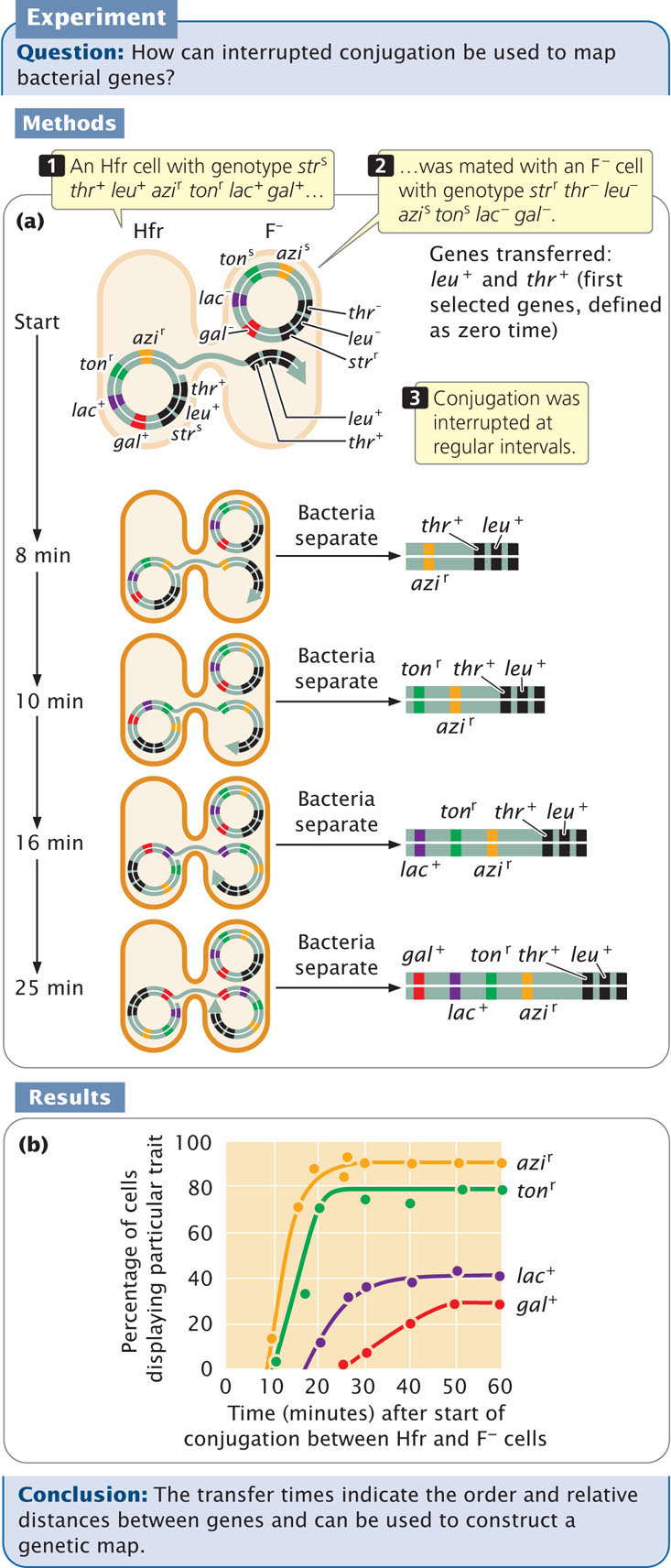
The two strains were mixed in nutrient medium and allowed to conjugate. After a few minutes, the medium was diluted to prevent any new pairings. At regular intervals, a sample of cells was removed and agitated vigorously in a kitchen blender to halt all conjugation and DNA transfer. The cells from each sample were plated on a selective medium that contained streptomycin and lacked leucine and threonine. The original donor cells were streptomycin sensitive (strs) and would not grow on this medium. The F− recipient cells were auxotrophic for leucine and threonine, and they also failed to grow on this medium. Only cells that underwent conjugation and received at least the leu+ and thr+ genes from the Hfr donors could grow on this medium. All strr leu+ thr+ cells were then tested for the presence of other genes that might have been transferred from the donor Hfr strain.
Because Jacob and Wollman used streptomycin to kill all the donor cells, they were not able to examine the transfer of the strs gene. All of the cells that grew on the selective medium were leu+ thr+; so we know that these genes were transferred. The percentage of strr leu+ thr+ cells receiving specific alleles (azir, tonr, leu+, and gal+) from the Hfr strain are plotted against the duration of conjugation (Figure 9.15b). What is the order in which the genes are transferred and the distances among them?
Solution Strategy
What information is required in your answer to the problem?
The order of the genes on the bacterial chromosome and the distances among them.
What information is provided to solve the problem?
 The donor cells were strs leu+ thr+ azir tonr lac+ gal+ and the recipient cells were strr leu− thr− azis tons lac− gal−.
The donor cells were strs leu+ thr+ azir tonr lac+ gal+ and the recipient cells were strr leu− thr− azis tons lac− gal−. The percentage of recipient cells with different traits that appear at various times after the start of conjugation (Figure 9.15).
The percentage of recipient cells with different traits that appear at various times after the start of conjugation (Figure 9.15).
Solution Steps
The first donor gene to appear in all of the recipient cells (at about 9 minutes) was azir. Gene tonr appeared next (after about 10 minutes), followed by lac+ (at about 18 minutes) and by gal+ (after 25 minutes). These transfer times indicate the order of gene transfer and the relative distances among the genes (see Figure 9.15b).
253
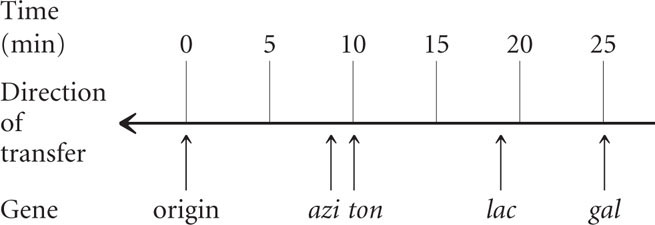
Notice that the frequency of gene transter from donor to recipient cells decreased for the more distant genes. For example, about 90% of the recipients received the azi1 allele, but only about 30% received the gal+ allele. The lower percentage for gal+ is due to the fact that some conjugating cells spontaneously broke apart before they were disrupted by the blender. The probability of spontaneous disruption increases with time, so fewer cells had an opportunity to receive genes that were transferred later.
For additional practice mapping bacterial genes with interrupted conjugation, try Problem 23 at the end of the chapter.
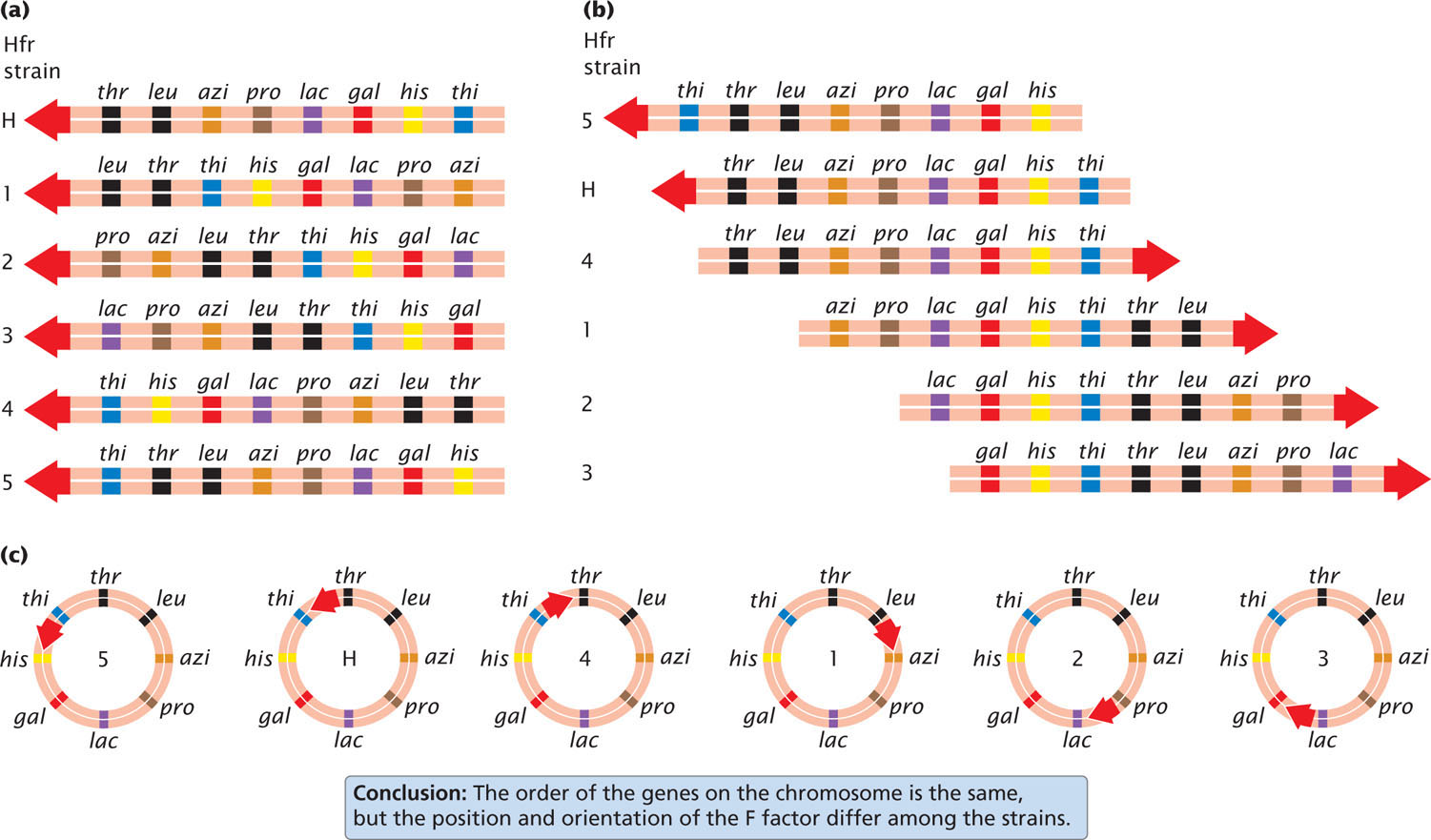
Directional Transfer and Mapping
Different Hfr strains of a given species of bacteria have the F factor integrated into the bacterial chromosome at different sites and in different orientations. Gene transfer always begins within F, and the orientation and position of F determine the direction and starting point of gene transfer. Figure 9.16a shows that, in strain Hfr1, F is integrated between leu and azi; the orientation of F at this site dictates that gene transfer will proceed in a counterclockwise direction around the circular chromosome. Genes from this strain will be transferred in the order of:
←leu-thr-thi-his-gal-lac-pro-azi
In strain Hfr5, F is integrated between the thi and the his genes (Figure 9.16b) and in the opposite orientation. Here gene transfer will proceed in a clockwise direction:
←thi-thr-leu-azi-pro-lac-gal-his
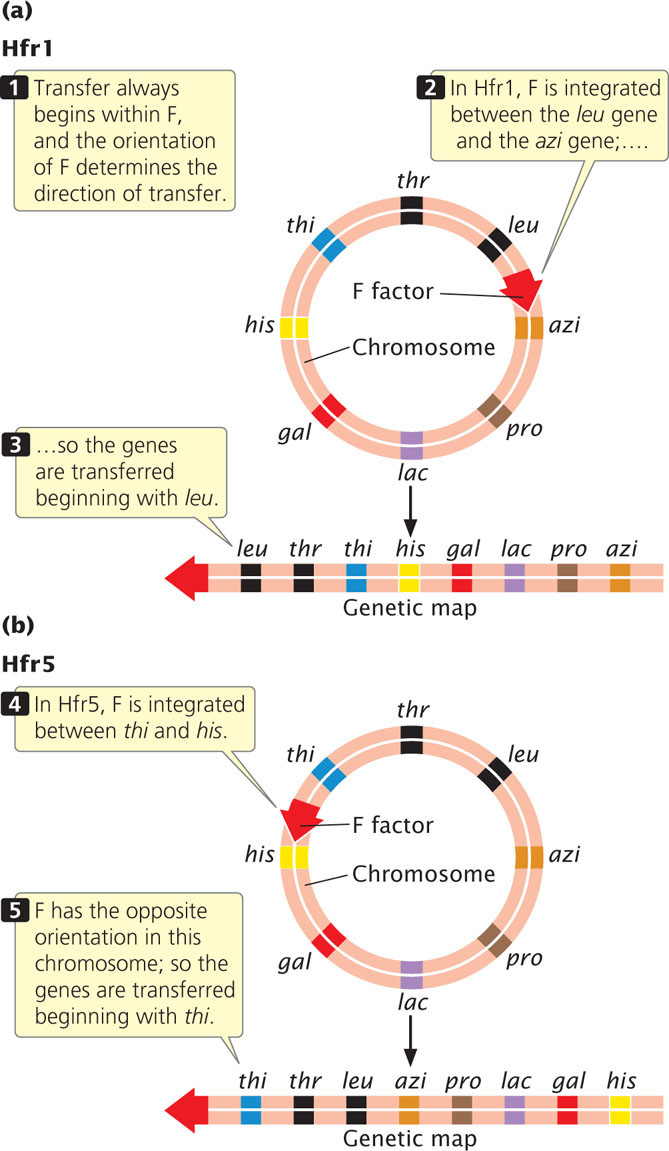
Although the starting point and direction of transfer may differ between two strains, the relative distance in time between any two pairs of genes is constant.
Notice that the order of gene transfer is not the same for different Hfr strains (Figure 9.17a). For example, azi is transferred just after leu in strain HfrH but long after leu in strain Hfr1. Aligning the sequences (Figure 9.17b) shows that the two genes on either side of azi are always the same: leu and pro. That they are the same makes sense when we recognize that the bacterial chromosome is circular and the starting point of transfer varies from strain to strain. These data provided the first evidence that the bacterial chromosome is circular (Figure 9.17c).  TRY PROBLEM 22
TRY PROBLEM 22
CONCEPTS
Conjugation can be used to map bacterial genes by mixing Hfr and F− cells of different genotypes and interrupting conjugation at regular intervals. The amount of time required for individual genes to be transferred from the Hfr to the F− cells indicates the relative positions of the genes on the bacterial chromosome.
 CONCEPT CHECK 4
CONCEPT CHECK 4
Interrupted conjugation was used to map three genes in E. coli. The donor genes first appeared in the recipient cells at the following times: gal, 10 minutes; his, 8 minutes; pro, 15 minutes. Which gene is in the middle?
254
Natural Gene Transfer and Antibiotic Resistance
Antibiotics are substances that kill bacteria. Their development and widespread use has greatly reduced the threat of infectious disease and saved countless lives. But many pathogenic bacteria have developed resistance to antibiotics, particularly in environments where antibiotics are routinely used, such as hospitals, livestock operations, and fish farms. In these environments where antibiotics are continually present, the only bacteria to survive are those that possess antibiotic resistance. No longer in competition with other bacteria, resistant bacteria multiply quickly and spread. In this way, the presence of antibiotics selects for resistant bacteria and reduces the effectiveness of antibiotic treatment for infections.
Antibiotic resistance in bacteria frequently results from the action of genes located on R plasmids, small circular plasmids that can be transferred by conjugation. Drug-resistant R plasmids have evolved in the past 60 years (since the beginning of widespread use of antibiotics), and some of them convey resistance to several antibiotics simultaneously. Ironic but plausible sources of some of the resistance genes found in R plasmids are the microbes that produce antibiotics in the first place. R plasmids can spread easily throughout the environment, passing between related and unrelated bacteria in a variety of common situations. For example, research shows that plasmids carrying genes for resistance to multiple antibiotics were transferred from a cow udder infected with E. coli to a human strain of E. coli on a hand towel: a farmer wiping his hands after milking an infected cow might unwittingly transfer antibiotic resistance from bovine- to human-inhabiting microbes. Conjugation taking place in minced meat on a cutting board allowed R plasmids to be passed from porcine (pig) to human E. coli. The transfer of R plasmids also takes place in sewage, soil, lake water, and marine sediments. The fact that R plasmids can easily spread throughout the environment and pass between related and unrelated bacteria underscores both the importance of limiting antibiotic use to the treatment of infections and the importance of hygiene in everyday life.
Transformation in Bacteria
A second way in which DNA can be transferred between bacteria is through transformation (see Figure 9.7b). Transformation played an important role in the initial identification of DNA as the genetic material, which will be discussed in Chapter 10.
255
Transformation requires both the uptake of DNA from the surrounding medium and its incorporation into a bacterial chromosome or a plasmid. It may occur naturally when dead bacteria break up and release DNA fragments into the environment. In soil and marine environments, this may be an important route of genetic exchange for some bacteria.
Mechanism Of Transformation
Cells that take up DNA through their membranes are said to be competent. Some species of bacteria take up DNA more easily than others: competence is influenced by growth stage, the concentration of available DNA in the environment, and other environmental factors. The DNA that a competent cell takes up need not be bacterial: virtually any type of DNA (bacterial or otherwise) can be taken up by competent cells under the appropriate conditions.
As a DNA fragment enters the cell in the course of transformation (Figure 9.18), one of the strands is broken up, whereas the other strand moves across the membrane and may pair with a homologous region and become integrated into the bacterial chromosome. This integration requires two crossover events, after which the remaining single-stranded DNA is degraded by bacterial enzymes. In some bacteria, double-stranded DNA moves across the cell membrane and is integrated into the bacterial chromosome.
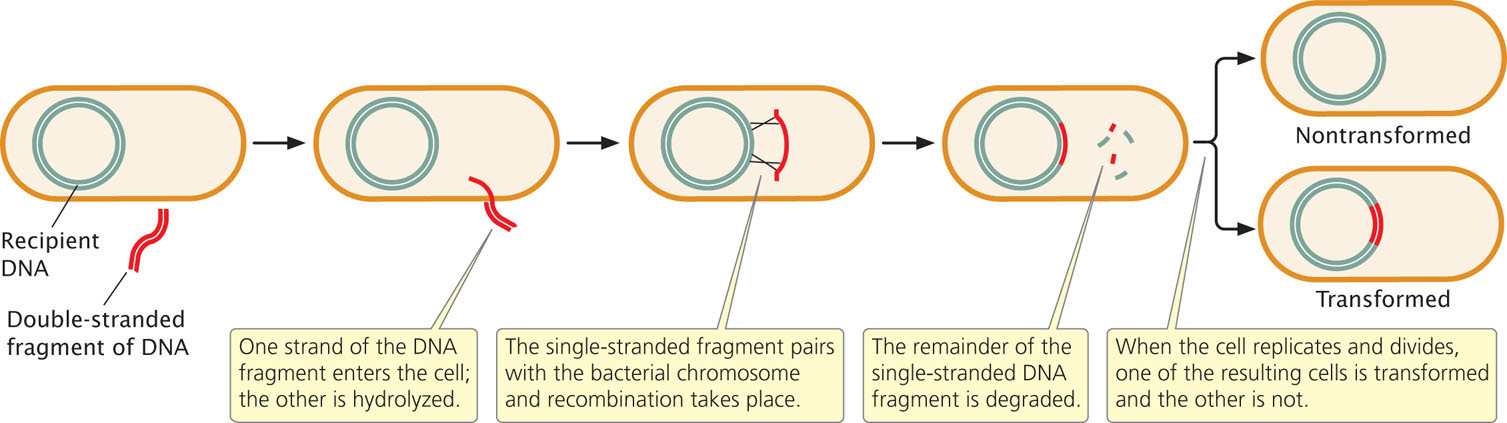
Bacterial geneticists have developed techniques for increasing the frequency of transformation in the laboratory in order to introduce particular DNA fragments into cells. They have also developed strains of bacteria that are more competent than wild-type cells. Treatment with calcium chloride, heat shock, or an electrical field makes bacterial membranes more porous and permeable to DNA, and the efficiency of transformation can also be increased by using high concentrations of DNA. These techniques enable researchers to transform bacteria such as E. coli, which are not naturally competent.
Gene Mapping with Transformation
Transformation, like conjugation, is used to map bacterial genes, especially in those species that do not undergo conjugation or transduction (see Figure 9.7a and c). Transformation mapping requires two strains of bacteria that differ in several genetic traits; for example, the recipient strain might be a− c− (auxotrophic for three nutrients), and the donor cell prototrophic with alleles a+ b+ c+ (Figure 9.19). DNA from the donor strain is isolated, purified, and fragmented. The recipient strain is treated to increase competency, and DNA from the donor strain is added to the medium. Fragments of the donor DNA enter the recipient cells and undergo recombination with homologous DNA sequences on the bacterial chromosome. Cells that receive genetic material through transformation are called transformants.
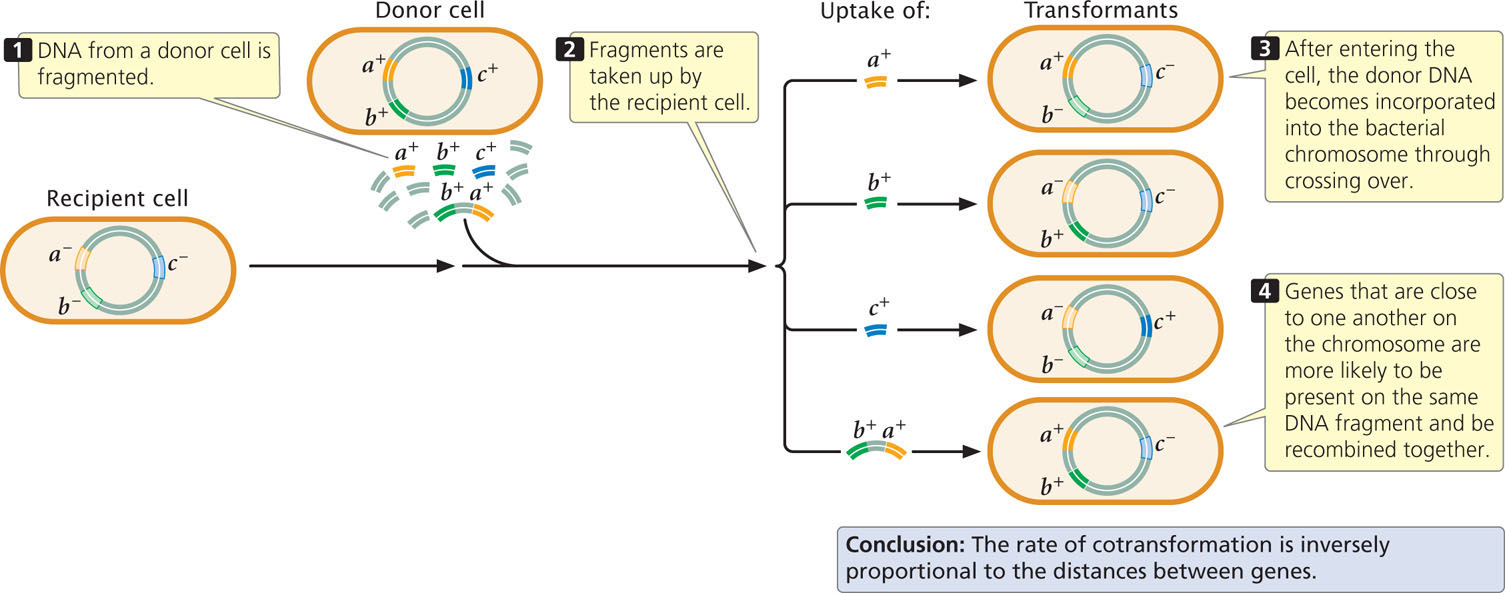
Genes can be mapped by observing the rate at which two or more genes are transferred to the host chromosome, or cotransformed, in transformation. When the donor DNA is fragmented before transformation, genes that are physically close on the chromosome are more likely to be present on the same DNA fragment and transferred together, as shown for genes a+ and b+ in Figure 9.19. Genes that are far apart are unlikely to be present on the same DNA fragment and will rarely be transferred together. Inside the cell, DNA becomes incorporated into the bacterial chromosome through recombination. If two genes are close together on the same fragment, any two crossovers are likely to take place on either side of the two genes, allowing both to become part of the recipient chromosome. If the two genes are far apart, there may be one crossover between them, allowing one gene but not the other to recombine with the bacterial chromosome. Thus, two genes are more likely to be transferred together when they are close together on the chromosome, and genes located far apart are rarely cotransformed. Therefore, the frequency of cotransformation can be used to map bacterial genes. If genes a and b as well as genes b and c are frequently cotransformed, but genes a and c are rarely cotransformed, then gene b must be between a and c—the gene order is a b c.  TRY PROBLEM 25
TRY PROBLEM 25
256
CONCEPTS
Genes can be mapped in bacteria by taking advantage of transformation—the ability of cells to take up DNA from the environment and incorporate it into their chromosomes through crossing over. The relative rate at which pairs of genes are cotransformed indicates the distance between them: the higher the rate of cotransformation, the closer the genes are on the bacterial chromosome.
 CONCEPT CHECK 5
CONCEPT CHECK 5
DNA from a bacterial strain with genotype his− leu− thr− is transformed with DNA from a strain that is his+ leu+ thr+. A few leu+ thr+ cells and a few his+ thr+ cells are found, but no his+ leu+ cells are observed. Which genes are farthest apart?
Bacterial Genome Sequences
Genetic maps serve as the foundation for more-detailed information provided by DNA sequencing, such as gene content and organization (see Chapter 19 for a discussion of gene sequencing). Geneticists have now determined the complete nucleotide sequences of more than two thousand bacterial genomes (see Table 20.1), and many additional microbial sequencing projects are underway.
Characteristics of Bacterial Genomes
Most bacterial genomes contain from 1 million to 4 million base pairs of DNA, but a few are much smaller (e.g., 580,000 bp in Mycoplasma genitalium) and some are considerably larger (e.g., more than 7 million bp in Mesorhizobium loti). The small size of bacterial genomes relative to those found in multicellular eukaryotes, which often have billions of base pairs of DNA, is thought to be an adaptation for rapid cell division, because the rate of cell division is limited by the time required to replicate the DNA. On the other hand, a lack of mobility in most bacteria requires metabolic and environmental flexibility, and so genome size and content are likely to be a balance between the opposing evolutionary forces of gene loss to maintain rapid reproduction and gene acquisition to ensure flexibility.
The function of a substantial proportion of genes in all bacteria has not been determined. Certain genes, particularly those with related functions, tend to reside next to one another, but these clusters are in very different locations in different species, suggesting that bacterial genomes are constantly being reshuffled. Comparisons of the gene sequences of disease-causing and benign bacteria are helping to identify genes implicated in disease and may suggest new targets for antibiotics and other antimicrobial agents.
Horizontal Gene Transfer
The availability of genome sequences has provided evidence that many bacteria have acquired genetic information from other species of bacteria—and sometimes even from eukaryotic organisms—in a process called horizontal gene transfer. In most eukaryotes, genes are passed only among members of the same species through the process of reproduction (called vertical transmission); that is, genes are passed down from one generation to the next. In horizontal transfer, genes can be passed between individual members of different species by nonreproductive mechanisms, such as conjugation, transformation, and transduction. Evidence suggests that horizontal gene transfer has taken place repeatedly among bacteria. For example, as much as 17% of E. coli’s genome has been acquired from other bacteria through horizontal gene transfer. Of medical significance, some pathogenic bacteria have acquired the genes necessary for infection, whereas others have acquired genes that confer resistance to antibiotics.
257
Because of the widespread occurence of horizontal gene transfer, many bacterial chromosomes are a mixture of genes inherited through vertical transmission and genes acquired through horizontal transfer. This situation has caused some biologists to question whether the species concept is even appropriate for bacteria. A species is often defined as a group of organisms that are reproductively isolated from other groups, have a set of genes in common, and evolve together (see Chapter 26). Because of horizontal gene transfer, the genes of one bacterial species are not isolated from the genes of other species, making the traditional species concept difficult to apply. Horizontal gene transfer also muddies the determination of the ancestral relationships among bacteria. The reconstruction of ancestrial relationships is usually based on genetic similarities and differences: organisms that are genetically similar are assumed to have descended from a recent common ancestor, whereas organisms that are genetically distinct are assumed to be more distantly related. Through horizontal gene transfer, however, even distantly related bacteria may have genes in common and thus appear to have descended from a recent common ancestor. The nature of species and how to classify bacteria are currently controversial topics within the field of microbiology.