9.3 Viruses Are Simple, Replicating Systems Amenable to Genetic Analysis
All organisms—plants, animals, fungi, and bacteria—are infected by viruses. A virus is a simple replicating structure made up of nucleic acid surrounded by a protein coat (see Figure 2.4). Viruses come in a great variety of shapes and sizes (Figure 9.20). Some have DNA as their genetic material, whereas others have RNA; the nucleic acid may be double stranded or single stranded, linear or circular.
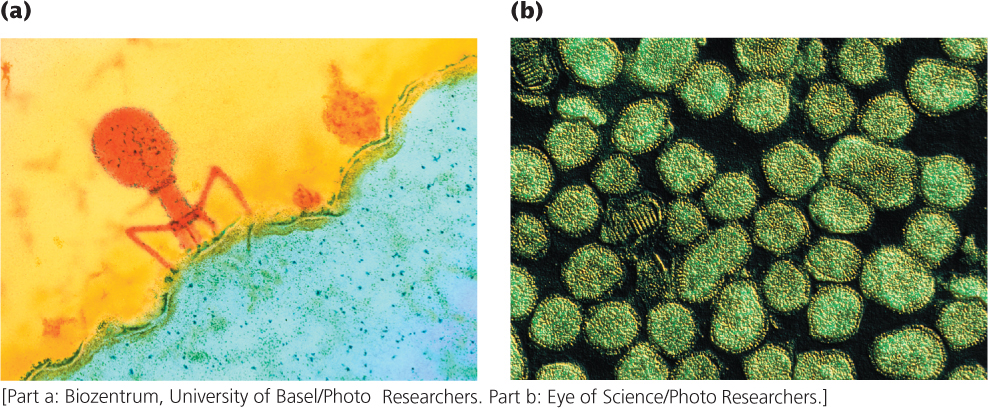
Viruses that infect bacteria (bacteriophages, or phages for short) have played a central role in genetic research since the late 1940s. They are ideal for many types of genetic research because they have small and easily manageable genomes, reproduce rapidly, and produce large numbers of progeny. Bacteriophages have two alternative life cycles: the lytic and the lysogenic cycles. In the lytic cycle, a phage attaches to a receptor on the bacterial cell wall and injects its DNA into the cell (Figure 9.21). Inside the host cell, the phage DNA is replicated, transcribed, and translated, producing more phage DNA and phage proteins. New phage particles are assembled from these components. The phages then produce an enzyme that breaks open the host cell, releasing the new phages. Virulent phages reproduce strictly through the lytic cycle and always kill their host cells.
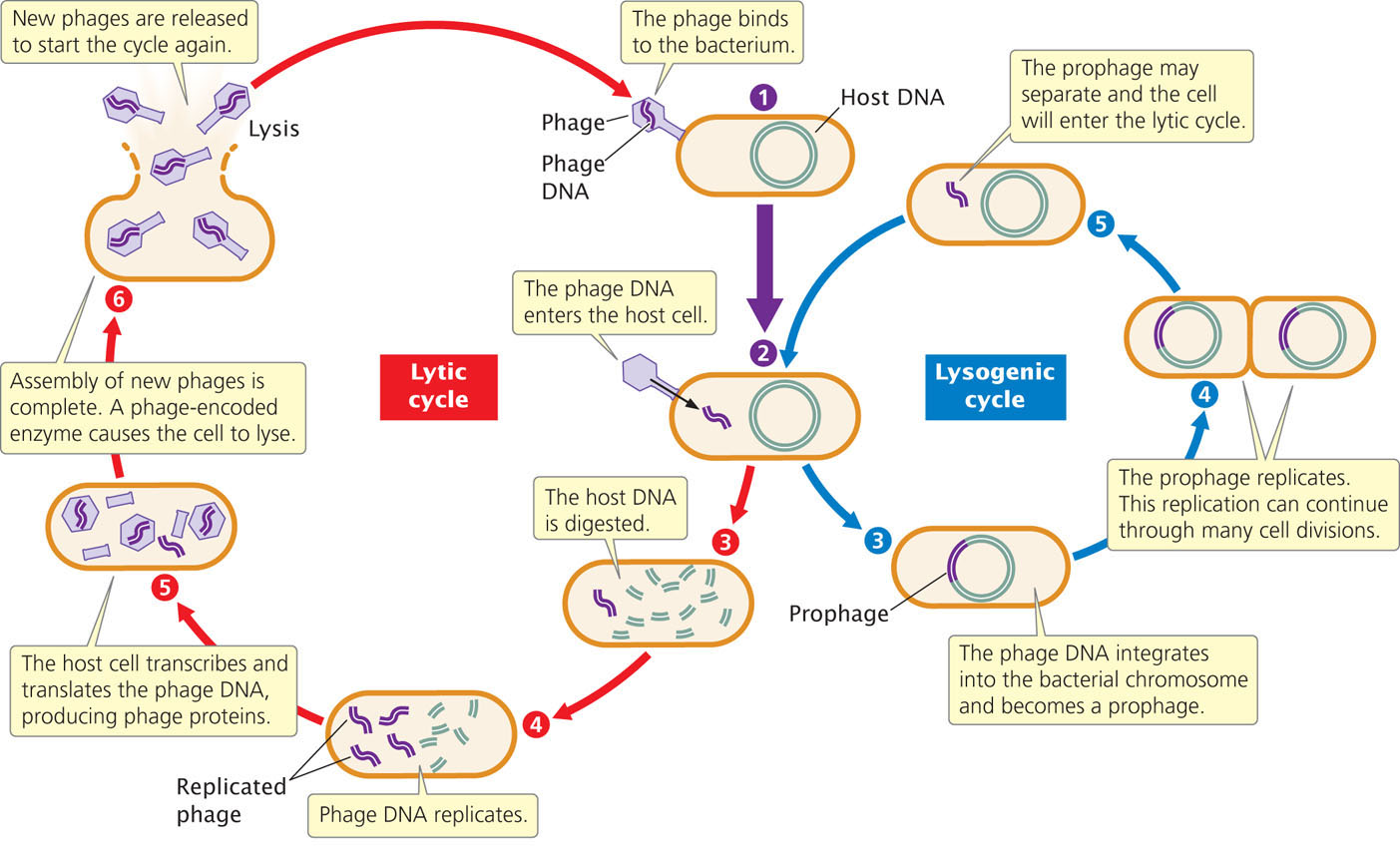
Temperate phages can undergo either the lytic or the lysogenic cycle. The lysogenic cycle begins like the lytic cycle (see Figure 9.21) but, inside the cell, the phage DNA integrates into the bacterial chromosome, where it remains as an inactive prophage. The prophage is replicated along with the bacterial DNA and is passed on when the bacterium divides. Certain stimuli can cause the prophage to dissociate from the bacterial chromosome and enter into the lytic cycle, producing new phage particles and lysing the cell.
Techniques for the Study of Bacteriophages
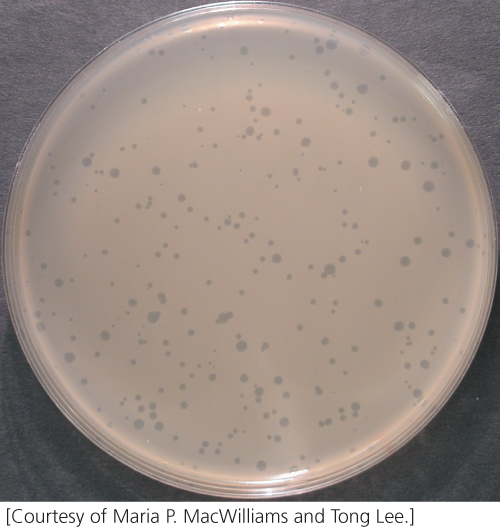
Viruses reproduce only within host cells so bacteriophages must be cultured in bacterial cells. Phages can be grown in large liquid cultures of bacteria to generate large numbers of offspring, but to study the characteristics of individual offspring we must isolate them on plates. We mix phages and bacteria together and plate them on solid medium on a petri plate. A high concentration of bacteria is used so that the colonies grow into one another and produce a continuous layer of bacteria, or “lawn,” on the agar. An individual phage infects a single bacterial cell and goes through its lytic cycle. Many new phages are released from the lysed cell and infect additional cells; the cycle is then repeated. Because the bacteria grow on solid medium the diffusion of the phages is restricted and only nearby cells are infected. After several rounds of phage reproduction, a clear patch of lysed cells, a plaque, appears on the plate (Figure 9.22). Each plaque represents a single phage that multiplied and lysed many cells. Plating a known volume of a dilute solution of phages on a bacterial lawn and counting the number of plaques that appear can be used to determine the original concentration of phage in the solution.
258
CONCEPTS
Viral genomes may be DNA or RNA, circular or linear, and double or single stranded. Bacteriophages are used in many types of genetic research.
 CONCEPT CHECK 6
CONCEPT CHECK 6
In which bacteriophage life cycle does the phage DNA become incorporated into the bacterial chromosome?
- Lytic
- Lysogenic
- Both lytic and lysogenic
- Neither lytic nor lysogenic
Transduction: Using Phages to Map Bacterial Genes
In the discussion of bacterial genetics, three mechanisms of gene transfer were identified: conjugation, transformation, and transduction (see Figure 9.7). Let’s take a closer look at transduction, in which genes are transferred between bacteria by viruses. In generalized transduction, any gene may be transferred. In specialized transduction, only a few genes are transferred.
259
Generalized Transduction
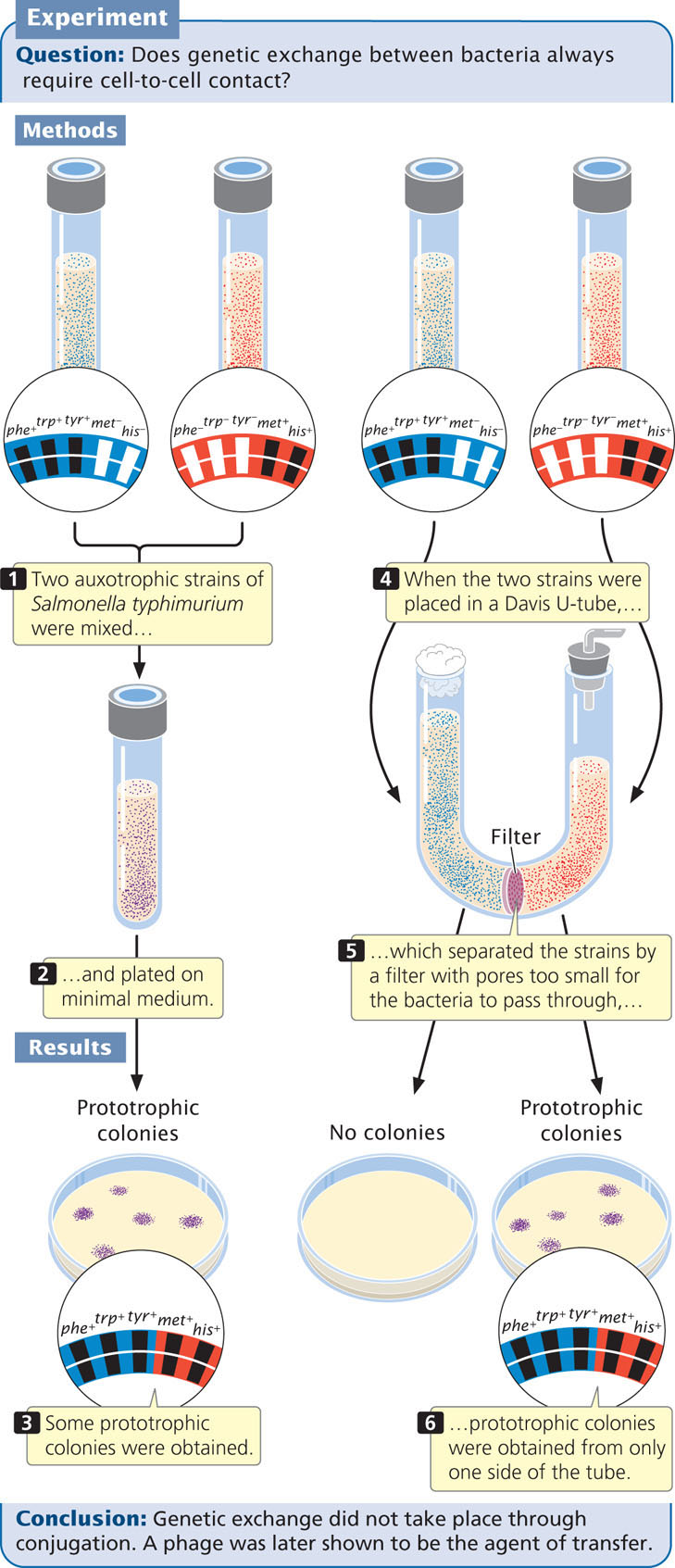
Joshua Lederberg and Norton Zinder discovered generalized transduction in 1952 while trying to produce recombination in the bacterium Salmonella typhimurium by conjugation. They mixed a strain of S. typhimurium that was phe+ trp+ tyr+ met− his− with a strain that was phe− trp− tyr− met+ his+ (Figure 9.23) and plated them on minimal medium. A few prototrophic recombinants (phe+ trp+ tyr+ met+ his+) appeared, suggesting that conjugation had taken place. However, when they tested the two strains in a U-shaped tube similar to the one used by Davis, some phe+ trp+ tyr+ met+ his+ prototrophs were obtained on one side of the tube (compare Figure 9.23 with Figure 9.9). This apparatus separated the two strains by a filter with pores too small for the passage of bacteria, so how were genes being transferred between bacteria in the absence of conjugation? The results of subsequent studies revealed that the agent of transfer was a bacteriophage.
In the lytic cycle of phage reproduction, the bacterial chromosome is broken into random fragments (Figure 9.24). For some types of bacteriophage, a piece of the bacterial chromosome instead of phage DNA occasionally gets packaged into a phage coat; these phage particles are called transducing phages. The transducing phage infects a new cell, releasing the bacterial DNA, and the introduced genes may then become integrated into the bacterial chromosome by a double crossover. Some transducing phages insert viral DNA, along with the bacterial gene, into the bacterial chromosome. In either case, bacterial genes can be moved from one bacterial strain to another, producing recombinant bacteria called transductants.
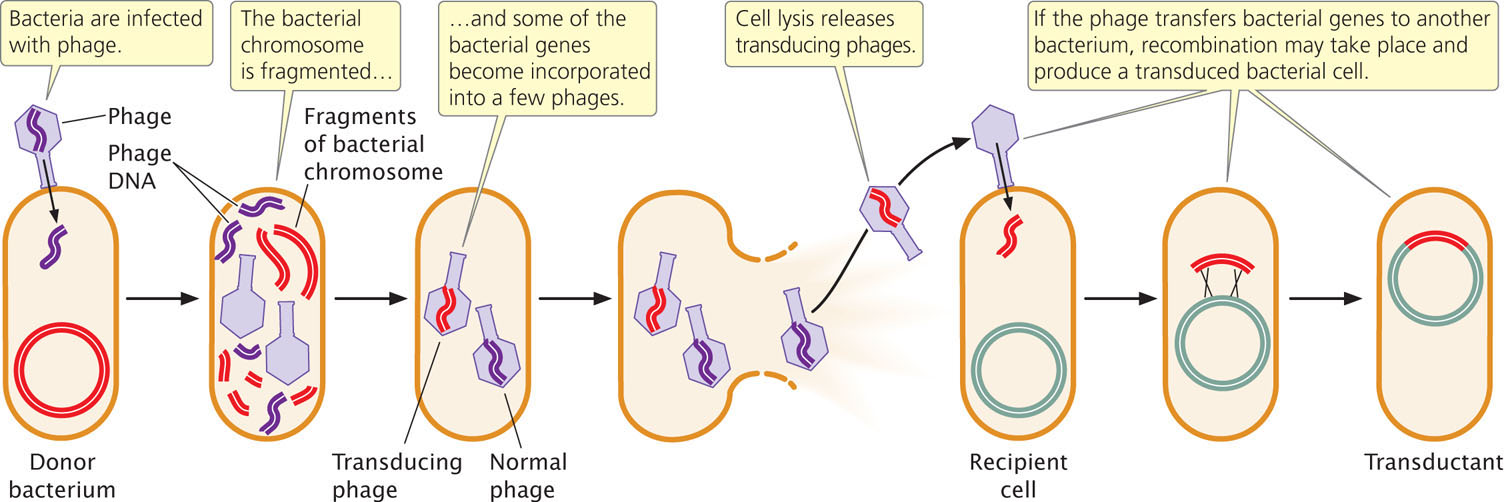
Not all phages are capable of transduction, a rare event that requires (1) that the phage degrade the bacterial chromosome, (2) that the process of packaging DNA into the phage protein not be specific for phage DNA, and (3) that the bacterial genes transferred by the virus recombine with the chromosome in the recipient cell. The overall rate of transduction ranges from only about 1 in 100,000 to 1 in 1,000,000.
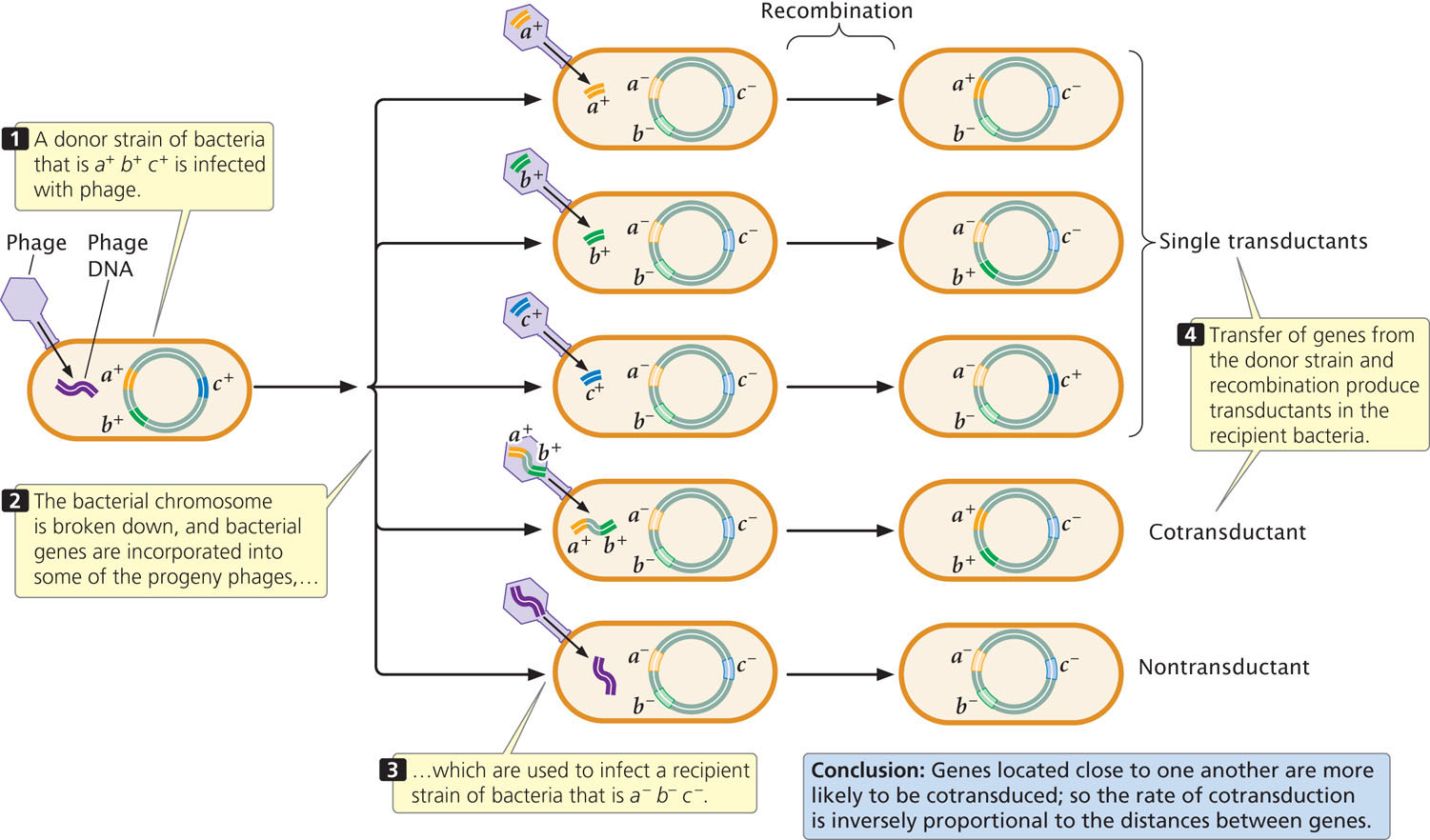
Because of the limited size of a phage particle, only about 1% of the bacterial chromosome can be transduced. Only genes located close together on the bacterial chromosome will be transferred together, or cotransduced. Because the chance of a cell being transduced by two separate phages is exceedingly small, any cotransduced genes are usually located close together on the bacterial chromosome. Thus, rates of cotransduction, like rates of cotransformation, give an indication of the physical distances between genes on a bacterial chromosome.
To map genes by using transduction, two bacterial strains with different alleles at several loci are used. The donor strain is infected with phages (Figure 9.25), which reproduce within the cell. When the phages have lysed the donor cells, a suspension of the progeny phages is mixed with a recipient strain of bacteria, which is then plated on several different kinds of media to determine the phenotypes of the transducing progeny phages.  TRY PROBLEM 32
TRY PROBLEM 32
260
261
CONCEPTS
In transduction, bacterial genes become packaged into a viral coat, are transferred to another bacterium by the virus, and become incorporated into the bacterial chromosome by crossing over. Bacterial genes can be mapped with the use of generalized transduction.
 CONCEPT CHECK 7
CONCEPT CHECK 7
In gene-mapping experiments using generalized transduction, bacterial genes that are cotransduced are
- far apart on the bacterial chromosome.
- on different bacterial chromosomes.
- close together on the bacterial chromosome.
- on a plasmid.
Specialized Transduction
Like generalized transduction, specialized transduction requires gene transfer from one bacterium to another through phages, but, here, only genes near particular sites on the bacterial chromosome are transferred. This process requires lysogenic bacteriophages. The prophage may imperfectly excise from the bacterial chromosome, carrying with it a small part of the bacterial DNA adjacent to the site of prophage integration. A phage carrying this DNA will then inject it into another bacterial cell in the next round of infection. This process resembles the situation in F′ cells, in which the F plasmid carries genes from one bacterium into another (see Figure 9.14). Specialized transduction occurs in phages that utilize specific integration sites on the bacterial chromosome; many phages integrate randomly and only exhibit generalized transduction.
CONCEPTS
Specialized transduction transfers only those bacterial genes located near the site of prophage insertion.
CONNECTING CONCEPTS: Three Methods for Mapping Bacterial Genes
Three methods of mapping bacterial genes have now been outlined: (1) interrupted conjugation; (2) transformation; and (3) transduction. These methods have important similarities and differences.
Mapping with interrupted conjugation is based on the time required for genes to be transferred from one bacterium to another by means of cell-to-cell contact. The key to this technique is that the bacterial chromosome itself is transferred, and the order of genes and the time required for their transfer provide information about the positions of the genes on the chromosome. In contrast with other mapping methods, the distance between genes is measured not in recombination frequencies but in units of time required for genes to be transferred. Here, the basic unit of conjugation mapping is a minute.
In gene mapping with transformation, DNA from the donor strain is isolated, broken up, and mixed with the recipient strain. Some fragments pass into the recipient cells, where the transformed DNA may recombine with the bacterial chromosome. The unit of transfer here is a random fragment of the chromosome. Loci that are close together on the donor chromosome tend to be on the same DNA fragment; so the rates of cotransformation provide information about the relative positions of genes on the chromosome.
Transduction mapping also relies on the transfer of genes between bacteria that differ in two or more traits, but, here, the vehicle of gene transfer is a bacteriophage. In a number of respects, transduction mapping is similar to transformation mapping. Small fragments of DNA are carried by the phage from donor to recipient bacteria, and the rates of cotransduction, like the rates of cotransformation, provide information about the relative distances between the genes.
All of the methods use a common strategy for mapping bacterial genes. The movement of genes from donor to recipient is detected by using strains that differ in two or more traits, and the transfer of one gene relative to the transfer of others is examined. Additionally, all three methods rely on recombination between the transferred DNA and the bacterial chromosome. In mapping with interrupted conjugation, the relative order and timing of gene transfer provide the information necessary to map the genes; in transformation and transduction, the rate of cotransfer provides this information.
In conclusion, the same basic strategies are used for mapping with interrupted conjugation, transformation, and transduction. The methods differ principally in their mechanisms of transfer: in conjugation mapping, DNA is transferred though contact between bacteria; in transformation, DNA is transferred as small naked fragments; and, in transduction, DNA is transferred by bacteriophages.
Gene Mapping in Phages
Mapping genes in the bacteriophages themselves depends on homologous recombination between phage chromosomes and therefore requires the application of the same principles as those applied to mapping genes in eukaryotic organisms (see Chapter 7). Crosses are made between viruses that differ in two or more genes, and recombinant progeny phages are identified and counted. The proportion of recombinant progeny is then used to estimate the distances between the genes and their linear order on the chromosome.
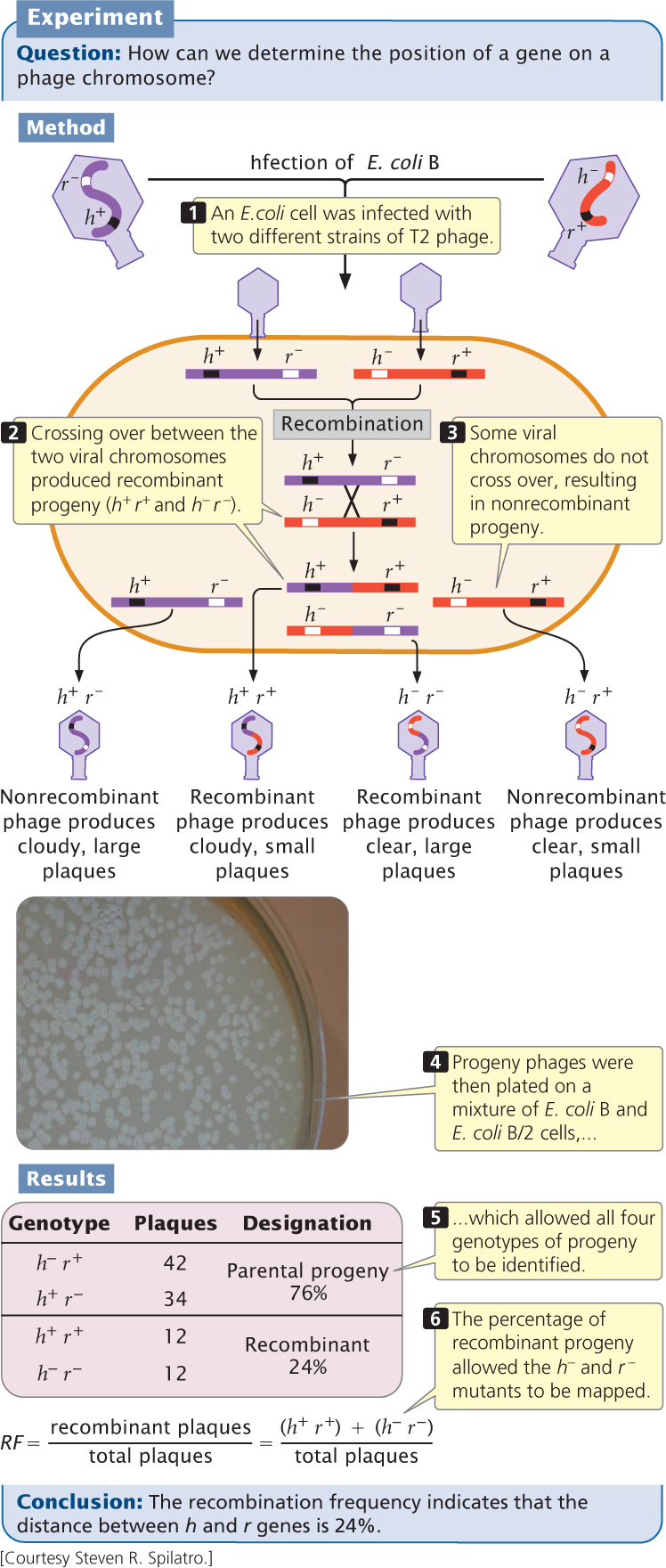
In 1949, Alfred Hershey and Raquel Rotman examined rates of recombination in the T2 bacteriophage, which has single-stranded DNA. They studied recombination between genes in two strains that differed in plaque appearance and host range (the bacterial strains that the phages could infect). One strain was able to infect and lyse type B E. coli cells but not B/2 cells (making this strain of phage wild type with normal host range, or h+) and produced an abnormal plaque that was large with distinct borders (r−). The other strain was able to infect and lyse both B and B/2 cells (mutant host range, h−) and produced wild-type plaques that were small with fuzzy borders (r+).
Hershey and Rotman crossed the h+ r− and h− r+ strains of T2 by infecting type B E. coli cells with a mixture of the two strains. They used a high concentration of phages so that most cells could be simultaneously infected by both strains (Figure 9.26). Within the bacterial cells, homologous recombination occasionally took place between the chromosomes of the different bacteriophage strains, producing h+ r+ and h− r− chromosomes, which were then packaged into new phage particles. When the cells lysed, the recombinant phages were released, along with the nonrecombinant h+ r− phages and h− r+ phages.
262
Hershey and Rotman diluted and plated the progeny phages on a bacterial lawn that consisted of a mixture of B and B/2 cells. Phages carrying the h+ allele (which conferred the ability to infect only B cells) produced a cloudy plaque because the B/2 cells did not lyse. Phages carrying the h− allele produced a clear plaque because all the cells within the plaque were lysed. The r+ phages produced small plaques, whereas the r− phages produced large plaques. The genotypes of these progeny phages could therefore be determined by the appearance of the plaque (see Figure 9.26 and Table 9.4).
| Phenotype | Genotype |
|---|---|
| Clear and small | h− r+ |
| Cloudy and large | h+ r− |
| Cloudy and small | h+ r+ |
| Clear and large | h− r− |
In this type of phage cross, the recombination frequency (RF) between the two genes can be calculated by using the following formula:
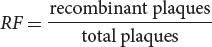
In Hershey and Rotman’s cross, the recombinant plaques were h+ r+ and h− r−; so the recombination frequency was
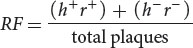
Recombination frequencies can be used to determine the distances between genes and their order on the phage chromosome, just as recombination frequencies are used to map genes in eukaryotes.  TRY PROBLEMS 31 AND 35
TRY PROBLEMS 31 AND 35
CONCEPTS
To map phage genes, bacterial cells are infected with viruses that differ in two or more genes. Recombinant plaques are counted, and rates of recombination are used to determine the linear order of the genes on the chromosome and the distances between them.
263
Fine-Structure Analysis of Bacteriophage Genes
In the 1950s and 1960s, Seymour Benzer conducted a series of experiments to examine the structure of a gene. Because no molecular techniques were available at the time for directly examining nucleotide sequences, Benzer was forced to infer gene structure from analyses of mutations and their effects. The results of his studies showed that different mutational sites within a single gene could be mapped (referred to as intragenic mapping) by using techniques similar to those described for mapping bacterial genes by transduction. Different sites within a single gene are very close together; so recombination between them takes place at a very low frequency. Because large numbers of progeny are required to detect these recombination events, Benzer used the bacteriophage T4, which reproduces rapidly and produces large numbers of progeny.
Benzer’s Mapping Techniques
Wild-type T4 phages normally produce small plaques with rough edges when grown on a lawn of E. coli. Certain mutants, called r for rapid lysis, produce larger plaques with sharply defined edges. Benzer isolated phages with a number of different r mutations, concentrating on one particular subgroup called rII mutants.
Wild-type T4 phages produce typical plaques (Figure 9.27) on E. coli strains B and K. In contrast, the rII mutants produce r plaques on strain B and do not form plaques at all on strain K. Benzer recognized the r mutants by their distinctive plaques when grown on E. coli B. He then collected lysate from these plaques and used it to infect E. coli K. Phages that did not produce plaques on E. coli K were defined as the rII type.

Benzer collected thousands of rII mutations. He simultaneously infected bacterial cells with two different mutants and looked for recombinant progeny (Figure 9.28). Consider two rII mutations, a− and b− (their wild-type alleles are a+ and b+). Benzer infected E. coli B cells with two different strains of phages, one a− b+ and the other a+ b− (see Figure 9.28, step 3). Neither of these mutations is able to grow on E. coli K cells. While reproducing within the B cells, a few phages of the two strains recombined (see Figure 9.28, step 4). A single crossover produces two recombinant chromosomes; one with genotype a+ b+ and the other with genotype a− b−:
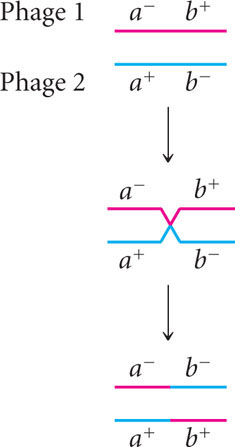
The resulting recombinant chromosomes, along with the nonrecombinant (parental) chromosomes, were incorporated into progeny phages, which were then used to infect E. coli K cells. The resulting plaques were examined to determine the genotype of the infecting phage and map the rII mutants (see Figure 9.28, step 5).
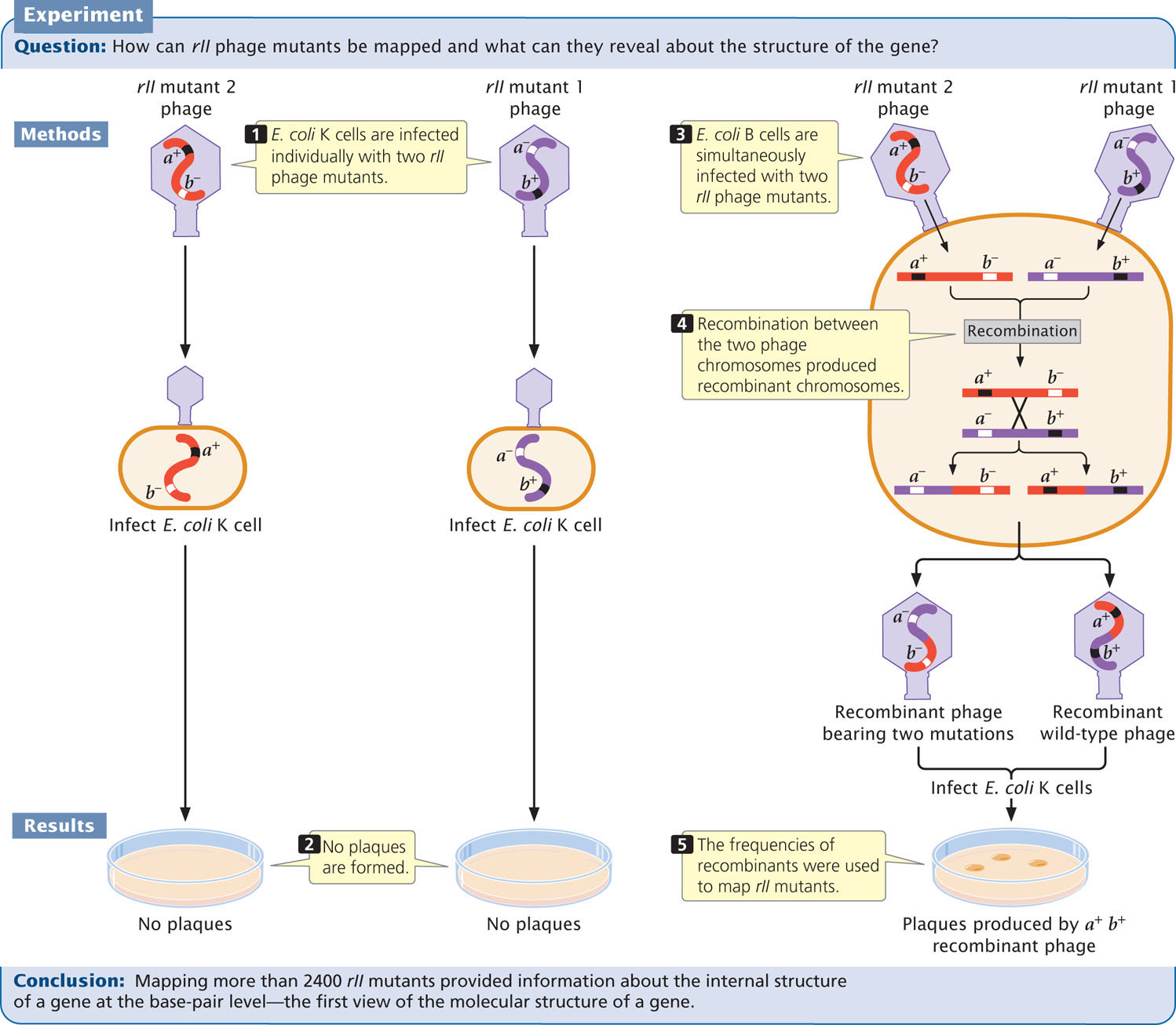
Neither of the rII mutants grew on E. coli K (see Figure 9.28, step 2), but wild-type phages grew; so progeny phages that produced plaques on E. coli K must have the recombinant genotype a+ b+. Each recombination event produces equal numbers of double mutants (a− b−) and wild-type chromosomes (a+ b+); so the number of recombinant progeny should be twice the number of wild-type plaques that appeared on E. coli K. The recombination frequency between the two rII mutants would be:
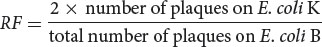
Because phages produce large numbers of progeny, Benzer was able to detect a single recombinant among billions of progeny phages. Recombination frequencies are proportional to physical distances along the chromosome, revealing the positions of the different mutations within the rII region of the phage chromosome. In this way, Benzer eventually mapped more than 2400 rII mutations, many corresponding to single base pairs in the viral DNA. His work provided the first molecular view of a gene.
Complementation Experiments
Benzer’s mapping experiments demonstrated that some rII mutations were very closely linked. This finding raised the question whether they were at the same locus or at different loci. To determine whether different rII mutations belonged to different functional loci, Benzer used the complementation (cis-trans) test.
264
To carry out the complementation test in bacteriophage, Benzer infected cells of E. coli K with large numbers of two mutant strains of phage (Figure 9.29, step 1) so that cells would become doubly infected with both strains. Consider two rII mutations: r101− and r104−. Cells infected with both mutants
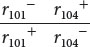
were effectively heterozygous for the phage genes, with the mutations in the trans configuration (see Figure 9.29, step 2). In the complementation testing, the phenotypes of progeny phages were examined on the K strain, rather than the B strain as illustrated in Figure 9.28.
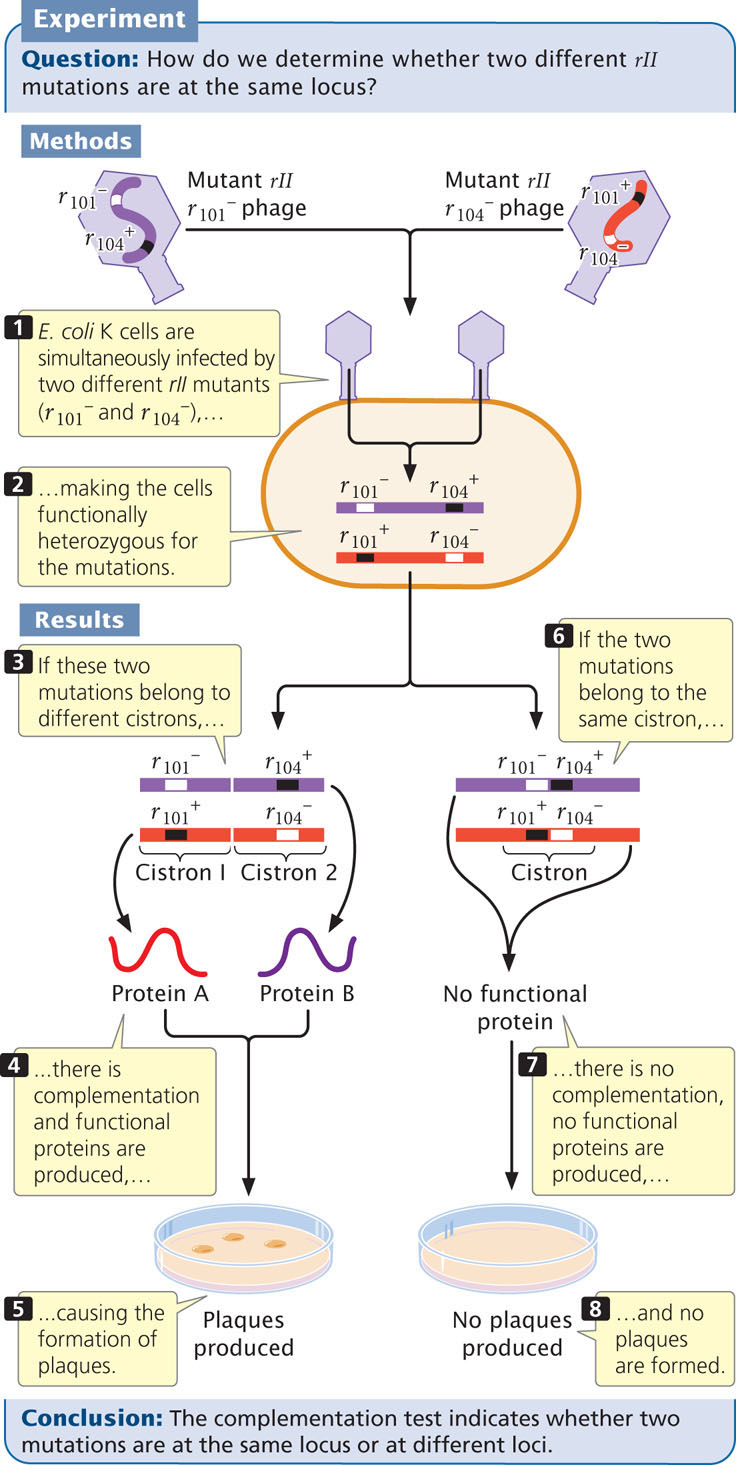
If the r101− and r104− mutations are at different functional loci that encode different proteins, then, in bacterial cells infected by both mutants, the wild-type sequences on the chromosome opposite each mutation will overcome the effects of the recessive mutations; the phages will produce normal plaques on E. coli K cells (Figure 9.29, steps 3, 4, and 5). If, on the other hand, the mutations are at the same locus, no functional protein is produced by either chromosome, and no plaques develop in the E. coli K cells (Figure 9.29, steps 6, 7, and 8). Thus, the absence of plaques indicates that the two mutations are at the same locus. Benzer coined the term cistron to designate a functional gene defined by the complementation test.
265
In the complementation test, the cis heterozygote is used as a control. Benzer simultaneously infected bacteria with wild-type phages  and with phages carrying both mutations
and with phages carrying both mutations  . This test produced cells that were heterozygous and in cis configuration for the phage genes:
. This test produced cells that were heterozygous and in cis configuration for the phage genes:
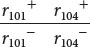
Regardless of whether the r101− and r104− mutations are in the same functional unit, these cells contain a copy of the wild-type phage chromosome  and will produce normal plaques in E. coli K.
and will produce normal plaques in E. coli K.
Benzer carried out complementation testing on many pairs of rII mutants. He found that the rII region consists of two loci, designated rIIA and rIIB. Mutations belonging to the rIIA and rIIB groups complemented each other, but mutations in the rIIA group did not complement others in rIIA; nor did mutations in the rIIB group complement others in rIIB.
CONCEPTS
In a series of experiments with the bacteriophage T4, Seymour Benzer showed that recombination could take place within a single gene and created the first molecular map of a gene. He used the complementation test to distinguish between functional genes (loci).
 CONCEPT CHECK 8
CONCEPT CHECK 8
In complementation tests, Benzer simultaneously infected E. coli cells with two phages, each of which carried a different mutation. What conclusion did he make when the progeny phages produced normal plaques?
- The mutations were at the same locus.
- The mutations were at different loci.
- The mutations were close together on the chromosome.
- The genes were in the cis configuration.
At the time of Benzer’s research, the relation between genes and DNA structure was unknown. A gene was defined as a functional unit of heredity that encoded a phenotype. Many geneticists believed that genes were indivisible and that recombination could not take place within them. Benzer demonstrated that intragenic recombination did indeed take place (although at a very low rate) and gave geneticists their first glimpse at the structure of an individual gene.  TRY PROBLEM 40
TRY PROBLEM 40
RNA Viruses
Thus far, we have primarily considered viruses that infect bacteria. Viruses also infect plants and animals, and some are important pathogens in these organisms. What we learned about bacteriophages has important implications for viruses that infect these more-complex organisms.
266
Viral genomes may be encoded in either DNA or RNA. RNA is the genetic material of some human viruses, including those that cause colds, influenza, polio, and AIDS. Almost all viruses that infect plants have RNA genomes. The medical and economic importance of RNA viruses has encouraged their study.
RNA viruses capable of integrating into the genomes of their hosts, much as temperate phages insert themselves into bacterial chromosomes, are called retroviruses (Figure 9.30a). Because the retroviral genome is RNA, whereas that of the host is DNA, a retrovirus must produce reverse transcriptase, an enzyme that synthesizes complementary DNA (cDNA) from either an RNA or a DNA template. A retrovirus uses reverse transcriptase to copy its RNA genome into a single-stranded DNA molecule, and the reverse transcriptase enzyme—or sometimes the host DNA polymerase—copies this single-stranded DNA, creating a double-stranded DNA molecule. The DNA copy of the viral genome then integrates into the host chromosome. A viral genome incorporated into the host chromosome is called a provirus. The provirus is replicated by host enzymes when the host chromosome is duplicated (Figure 9.30b).
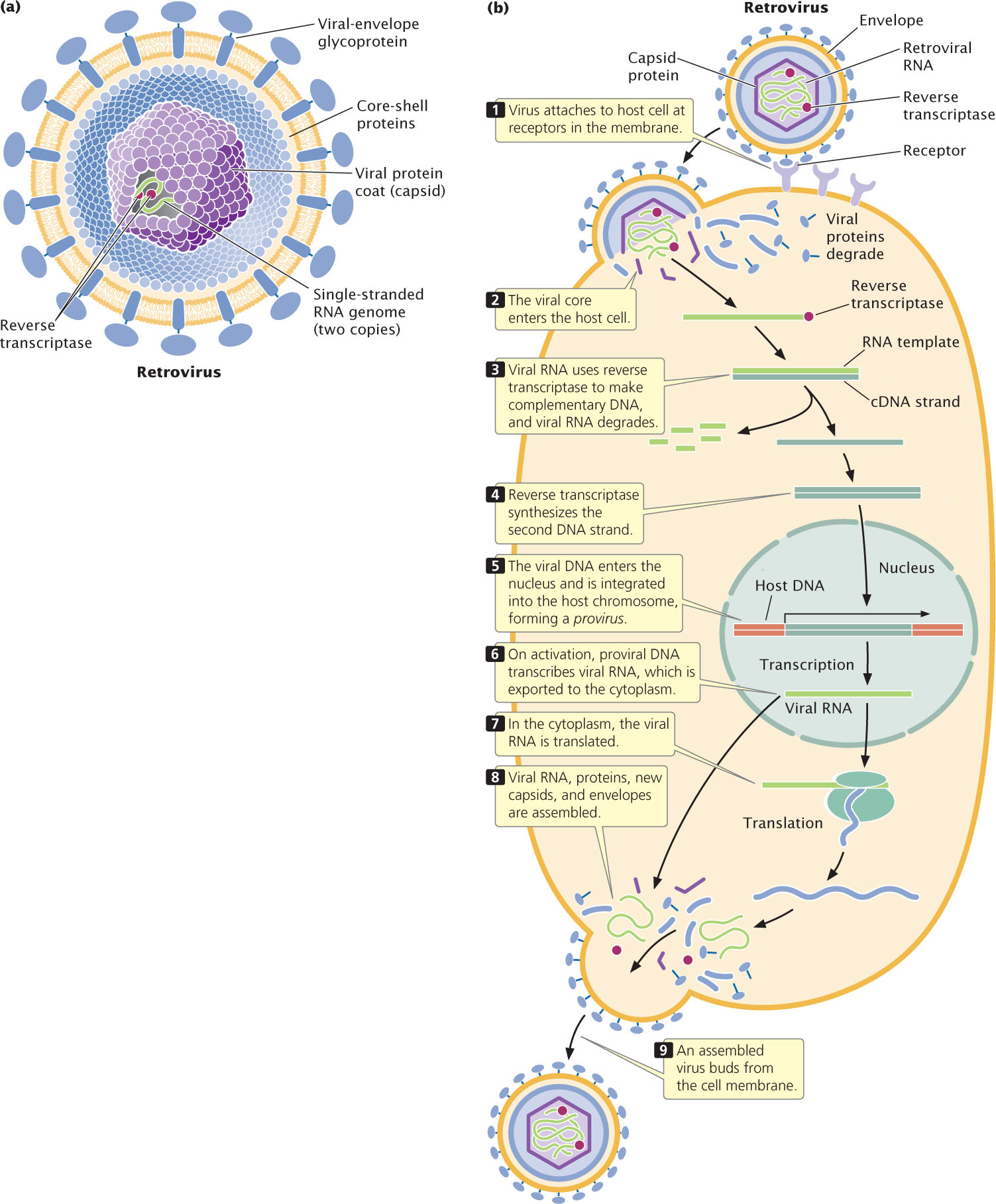
When conditions are appropriate, the provirus undergoes transcription to produce numerous copies of the original viral RNA genome. This RNA encodes viral proteins and serves as genomic RNA for new viral particles. As these viruses escape the cell, they collect patches of the cell membrane to use as their envelopes.
All known retroviral genomes have three genes in common: gag, pol, and env, each encoding a precursor protein that is cleaved into two or more functional proteins. The gag gene encodes proteins that make up the viral protein coat. The pol gene encodes reverse transcriptase and an enzyme called integrase, which inserts the viral DNA into the host chromosome. The env gene encodes the glycoproteins that appear on the surface of the virus.
267
Some retroviruses contain oncogenes (see Chapter 23) that may stimulate cell division and cause the formation of tumors. The first retrovirus to be isolated, the Rous sarcoma virus, was originally recognized by its ability to produce connective-tissue tumors (sarcomas) in chickens.
Human Immunodeficiency Virus and AIDS
An example of a retrovirus is human immunodeficiency virus (HIV), which causes acquired immune deficiency syndrome (AIDS). AIDS was first recognized in 1982, when a number of homosexual males in the United States began to exhibit symptoms of a new immune-system-deficiency disease. In that year, Robert Gallo proposed that AIDS was caused by a retrovirus. Between 1983 and 1984, as the AIDS epidemic became widespread, the HIV retrovirus was isolated from AIDS patients. AIDS is now known to be caused by two different immunodeficiency viruses, HIV-1 and HIV-2, which together have infected more than 60 million people worldwide. Of those infected, 30 million have died. Most cases of AIDS are caused by HIV-1, which now has a global distribution; HIV-2 is found primarily in western Africa.
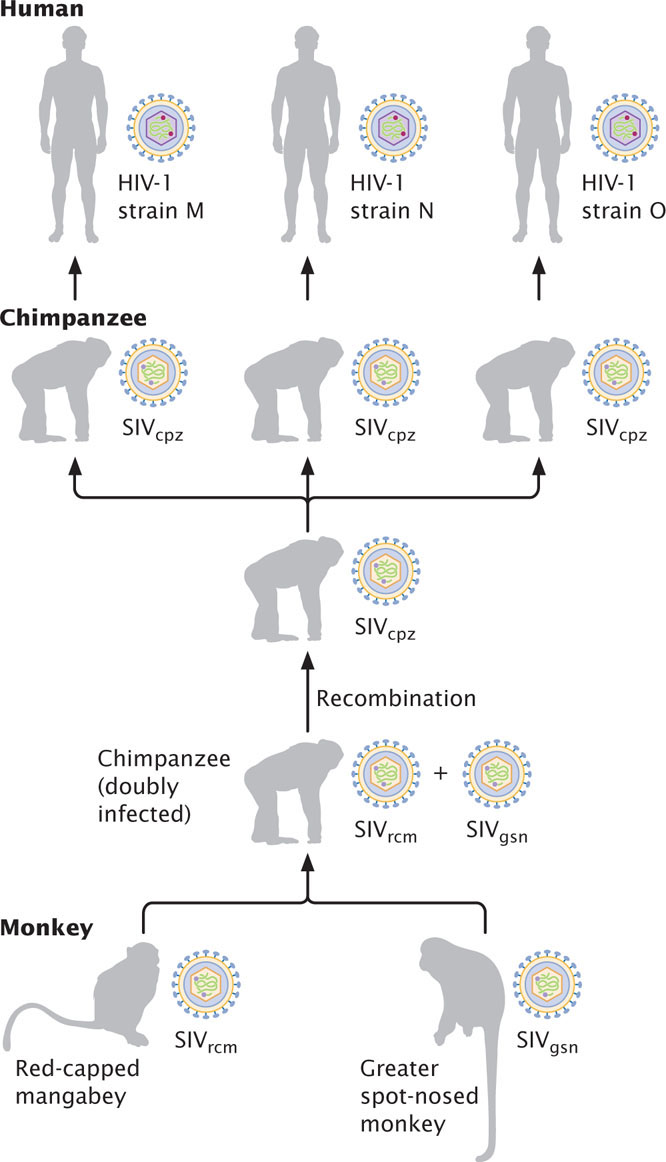
HIV illustrates the importance of genetic recombination in viral evolution. Studies of the DNA sequences of HIV and other retroviruses reveal that HIV-1 is closely related to the simian immunodeficiency virus found in chimpanzees (SIVcpz). Many wild chimpanzees in Africa are infected with SIVcpz, although it doesn’t cause AIDS-like symptoms in chimps. SIVcpz is itself a hybrid that resulted from recombination between a retrovirus found in the red-capped mangabey (a monkey) and a retrovirus found in the greater spot-nosed monkey (Figure 9.31). Apparently, one or more chimpanzees became infected with both viruses; recombination between the viruses produced SIVcpz, which was then transmitted to humans through contact with infected chimpanzees. In humans, SIVcpz underwent significant evolution to become HIV-1, which then spread throughout the world to produce the AIDS epidemic. Several independent transfers of SIVcpz to humans gave rise to different strains of HIV-1. HIV-2 evolved from a different retrovirus, SIVsm, found in sooty mangabeys.
HIV is transmitted by sexual contact between humans and through any type of blood-to-blood contact, such as that caused by the sharing of dirty needles by drug addicts. HIV can also be transmitted between mother and child during pregnancy and after pregnancy in breast milk. Until screening tests could identify HIV-infected blood, transfusions and clotting factors used by hemophiliacs were sources of infection as well.
HIV principally attacks a class of blood cells called helper T lymphocytes or, simply, helper T cells (Figure 9.32). HIV enters a helper T cell, undergoes reverse transcription, and integrates into the chromosome. The virus reproduces rapidly, destroying the T cell as new virus particles escape from the cell. Because helper T cells are central to immune function and are destroyed in the infection, AIDS patients have a diminished immune response; most AIDS patients die of secondary infections that develop because they have lost the ability to fight off pathogens.
The HIV genome is 9749 nucleotides long and carries gag, pol, env, and six other genes that regulate the life cycle of the virus. HIV’s reverse transcriptase is very error prone, giving the virus a high mutation rate and allowing it to evolve rapidly, even within a single host. This rapid evolution makes the development of an effective vaccine against HIV particularly difficult. Genetic variation within the human population also affects the virus. To date, more than 10 loci in humans that affect HIV infection and the progression of AIDS have been identified.
268
CONCEPTS
A retrovirus is an RNA virus that integrates into its host’s chromosome by making a DNA copy of its RNA genome through the process of reverse transcription. Human immunodeficiency virus, the causative agent of AIDS, is a retrovirus. It evolved from related retroviruses found in other primates.
 CONCEPT CHECK 9
CONCEPT CHECK 9
What enzyme is used by a retrovirus to make a DNA copy of its genome?
Influenza
Influenza demonstrates how rapid changes in a pathogen can arise through recombination of its genetic material. Influenza, commonly called flu, is a respiratory disease caused by influenza viruses. In the United States, from 5% to 20% of the entire population is infected with influenza annually and, though most cases are mild, an estimated 36,000 people die from influenza-related causes each year. At certain times, particularly when new strains of influenza virus enter the human population, there are worldwide epidemics (called pandemics); for example, in 1918, the Spanish flu virus killed an estimated 20 million to 100 million people worldwide.
Influenza viruses are RNA viruses that infect birds and mammals. The three main types are influenza A, influenza B, and influenza C. Most cases of the common flu are caused by influenza A and B. Influenza A is divided into subtypes on the basis of two proteins, hemagglutinin (HA) and neuraminidase (NA), found on the surface of the virus. The HA and NA proteins affect the ability of the virus to enter host cells and the host organism’s immune response to infection. There are 16 types of HA and 9 types of NA, which can exist in a virus in different combinations. For example, common strains of influenza A circulating in humans today are H1N1 and H3N2 (Table 9.5), along with several strains of influenza B. Most of the different subtypes of influenza A are found in birds.
| Year | Influenza Pandemic | Strain |
|---|---|---|
| 1918 | Spanish flu | H1N1 |
| 1957 | Asian flu | H2N2 |
| 1968 | Hong Kong flu | H3N2 |
| 2009 | Swine flu | H1N1 |
Although influenza is an RNA virus, it is not a retrovirus: its genome is not copied into DNA and incorporated into the host chromosome as is that of a retrovirus. The influenza viral genome consists of seven or eight pieces of RNA that are enclosed in a viral envelope. Each piece of RNA encodes one or two of the virus’s proteins. The virus enters a host cell by attaching to specific receptors on the cell membrane. After the viral particle has entered the cell, the viral RNA is released, copied, and translated into viral proteins. Viral RNA molecules and viral proteins are then assembled into new viral particles, which exit the cell and infect additional cells.
One of the dangers of the influenza virus is that it evolves rapidly, with new strains appearing frequently. Influenza evolves in two ways. First, each strain continually changes through mutations arising in the viral RNA. The enzyme that copies the RNA is especially prone to making mistakes, and so new mutations are continually introduced into the viral genome. This type of continual change is called antigenic drift. Occasionally, major changes in the viral genome take place through antigenic shift, in which genetic material from different strains is combined in a process called reassortment. Reassortment takes place when a host is simultaneously infected with two different strains. The RNAs of both strains are replicated within the cell, and RNA segments from two different strains are incorporated into the same viral particle, creating a new strain. For example, in 2002, reassortment occurred between the H1N1 and H3N2 subtypes, creating a new H1N2 strain that contained the hemagglutinin from H1N1 and the neuraminidase from H3N2. New strains produced by antigenic shift are responsible for most pandemics, because no one has immunity to the radically different virus that is produced.
269
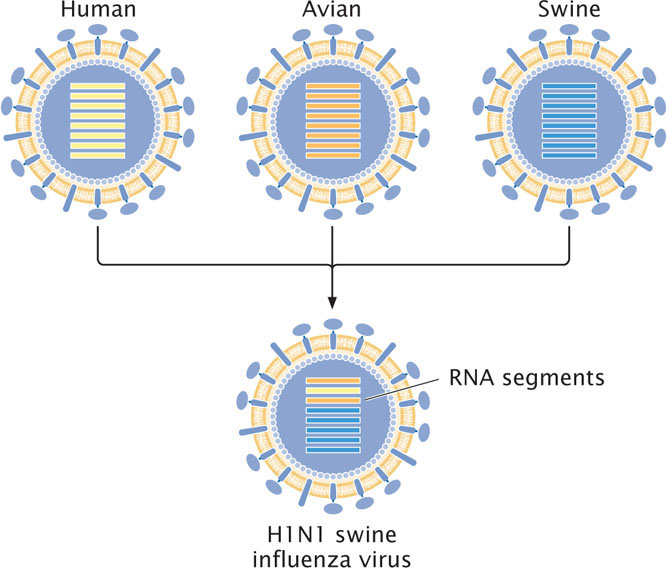
Most different strains of influenza A infect birds. Humans are not easily infected with bird influenza. The appearance of new strains in humans is thought to often arise from viruses that reassort in pigs, which can be infected by viruses from both humans and birds. In 2009, a new strain of H1N1 influenza (called swine flu) emerged in Mexico and quickly spread throughout the world. This virus arose from a series of reassortment events that combined gene sequences from human, bird, and pig influenza viruses to produce the new H1N1 virus (Figure 9.33). Farming practices that raise pigs and birds in close proximity may facilitate reassortment among avian, swine, and human strains of influenza.
CONCEPTS
Influenza is caused by RNA influenza viruses. New strains of influenza appear through antigenic shift, in which new viral genomes are created through the reassortment of RNA molecules of different strains.