Elongation
The next stage in protein synthesis is elongation, in which amino acids are joined to create a polypeptide chain. Elongation requires (1) the 70S initiation complex just described; (2) tRNAs charged with their amino acids; (3) several elongation factors; and (4) GTP.
A ribosome has three sites that can be occupied by tRNAs: the aminoacyl (A) site, the peptidyl (P) site, and the exit (E) site (Figure 11.11a). The initiator tRNA immediately occupies the P site (the only site to which the fMet-
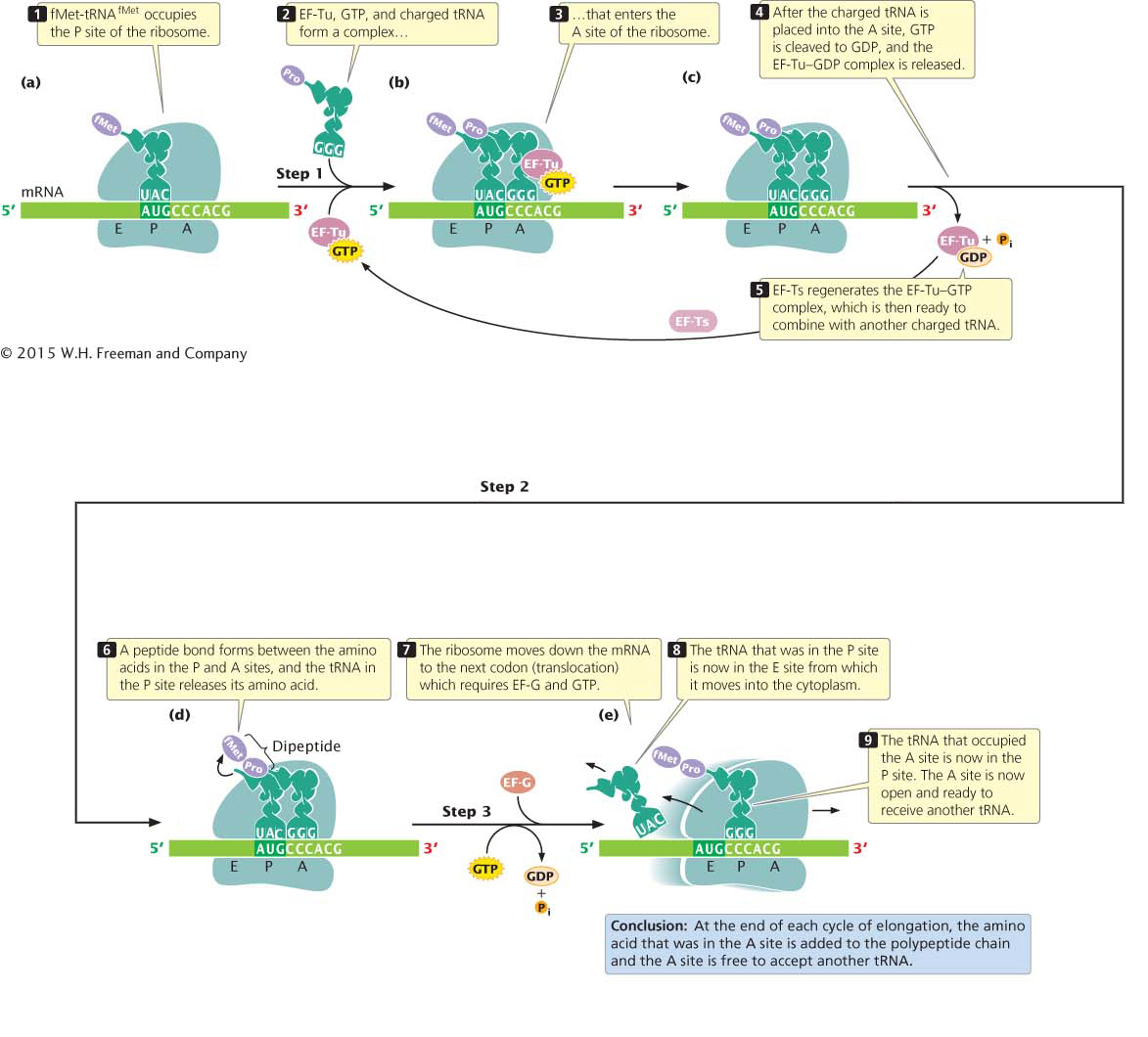
Elongation takes place in three steps. In the first step (Figure 11.11b), a charged tRNA binds to the A site. This binding takes place when elongation factor Tu (EF-
The second step of elongation is the formation of a peptide bond between the amino acids that are attached to tRNAs in the P and A sites (Figure 11.11d). The formation of this peptide bond releases the amino acid in the P site from its tRNA. Peptide-
The third step in elongation is translocation (Figure 11.11e), the movement of the ribosome down the mRNA in the 5′ → 3′ direction. This step positions the ribosome over the next codon and requires elongation factor G (EF-
299
After translocation, the A site of the ribosome is empty and ready to receive the tRNA specified by the next codon. The elongation cycle (see Figure 11.11b through e) repeats itself: a charged tRNA and its amino acid occupy the A site, a peptide bond is formed between the amino acids in the A and P sites, and the ribosome translocates to the next codon. Throughout the cycle, the polypeptide chain remains attached to the tRNA in the P site.
Elongation in eukaryotic cells takes place in a similar manner. Eukaryotes possess at least three elongation factors, one of which also acts in initiation and termination. Another of these elongation factors, called eukaryotic elongation factor 2 (eEF2), is the target of a toxin produced by the bacteria that cause diphtheria, a disease that until recently was a leading killer of children. The diphtheria toxin inhibits eEF2, preventing the translocation of the ribosome along the mRNA, and protein synthesis ceases.
CONCEPTS
Elongation consists of three steps: (1) a charged tRNA enters the A site, (2) a peptide bond is created between amino acids in the A and P sites, and (3) the ribosome translocates to the next codon. Elongation requires several elongation factors and GTP.
 CONCEPT CHECK 6
CONCEPT CHECK 6
In elongation, the creation of peptide bonds between amino acids is catalyzed by
rRNA.
protein in the small subunit.
protein in the large subunit.
tRNA.
a