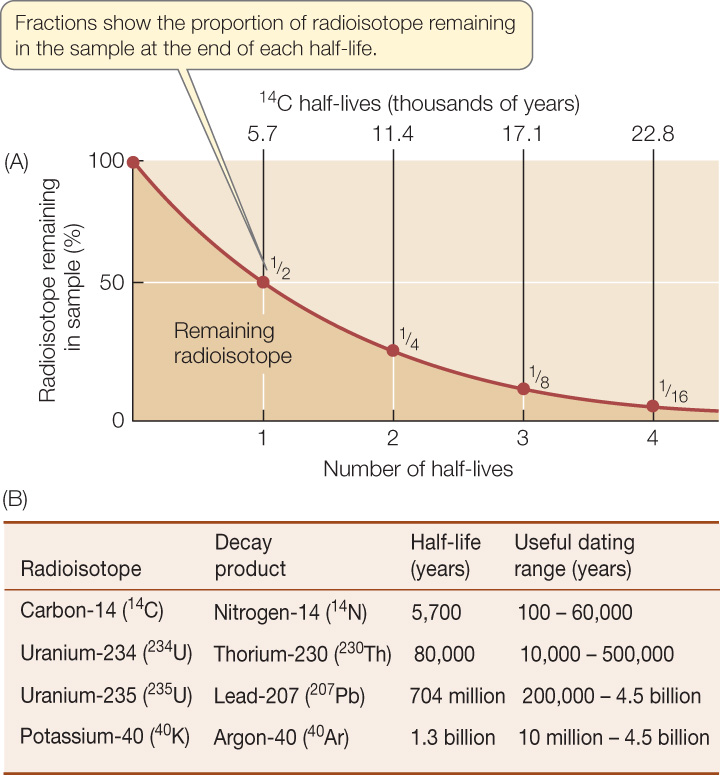
Figure 18.1: Radioactive Isotopes Allow Us to Date Ancient Rocks The decay of radioactive isotopes into stable isotopes happens at a steady rate. A half-life is the time it takes for half of the remaining atoms to decay in this way. (A) The graph demonstrates the principle of half-life using carbon-14 (14C) as an example. The half-life of 14C is 5,700 years. (B) Examples of some radioisotopes with different characteristic half-lives that allow us to estimate the ages of many rocks.