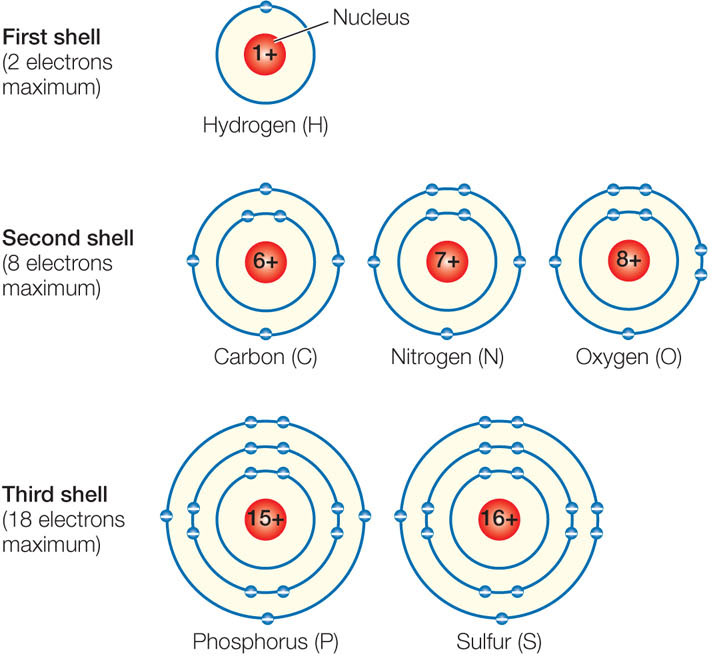
Figure 2.1: Electron Shells Each shell can hold a specific maximum number of electrons and must be filled before electrons can occupy the next shell. The energy level of an electron is higher in a shell farther from the nucleus. An atom with fewer than eight electrons in its outermost shell (or two in the case of hydrogen) can react (bond) with other atoms.