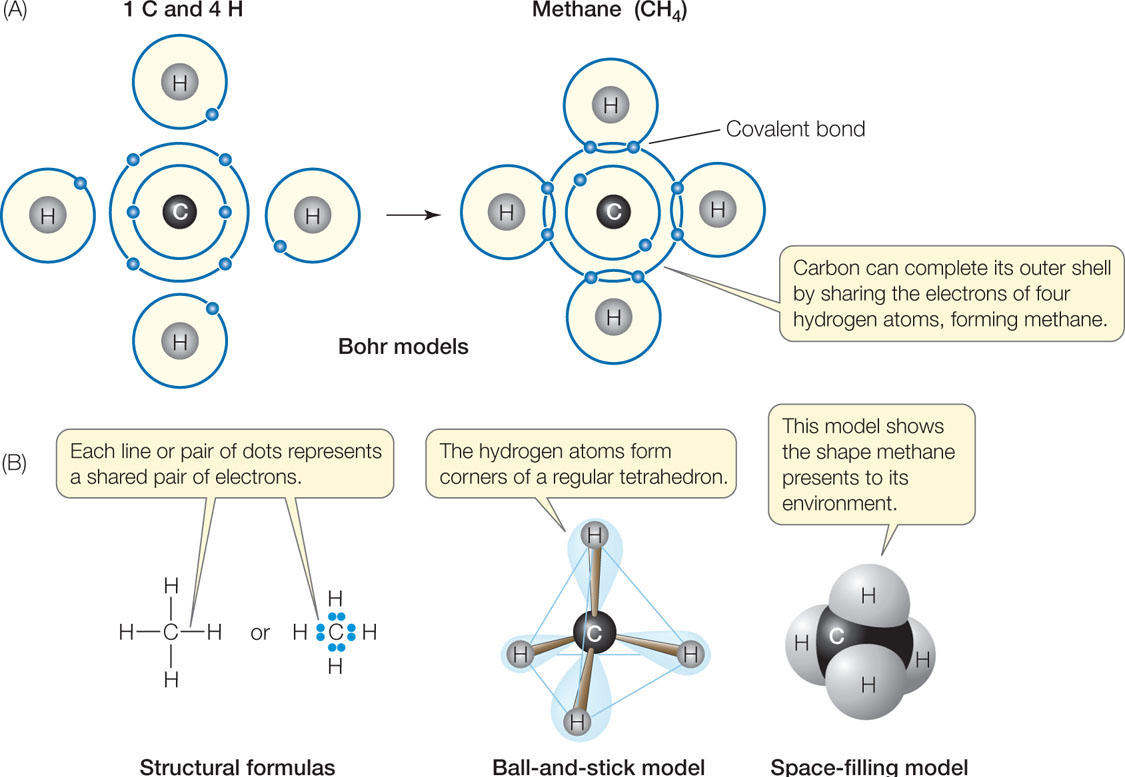
Figure 2.3: Covalent Bonding (A) Bohr models showing the formation of covalent bonds in methane, whose molecular formula is CH4. Electrons are shown in shells around the nuclei. (B) Three additional ways of representing the structure of methane. The ball-and-stick and the spacefilling models show the spatial orientations of the bonds. The spacefilling model indicates the overall shape and surface of the molecule. In the chapters that follow, different conventions will be used to depict molecules. Bear in mind that these are models to illustrate certain properties and are not the most accurate portrayals of reality.