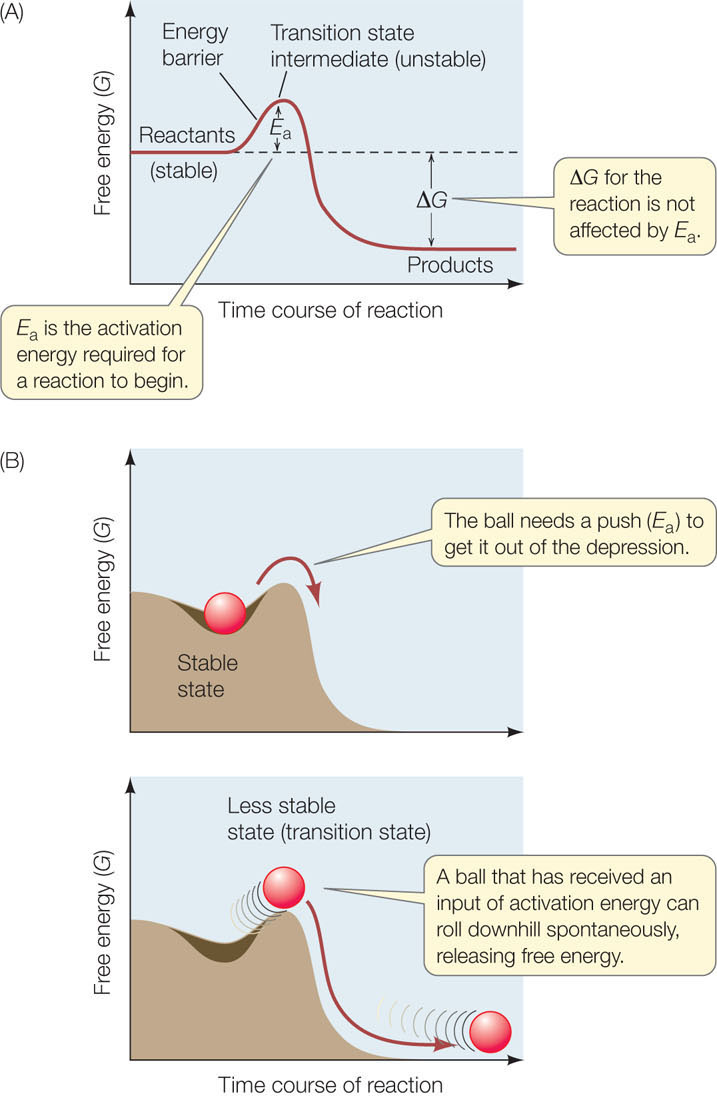
Figure 3.12: Activation Energy Initiates Reactions (A) In any chemical reaction, an initial stable state must become less stable before change is possible. (B) A ball on a hillside provides a physical analogy to the biochemical principle graphed in A. Although these graphs show an exergonic reaction, activation energy is needed for endergonic reactions as well.