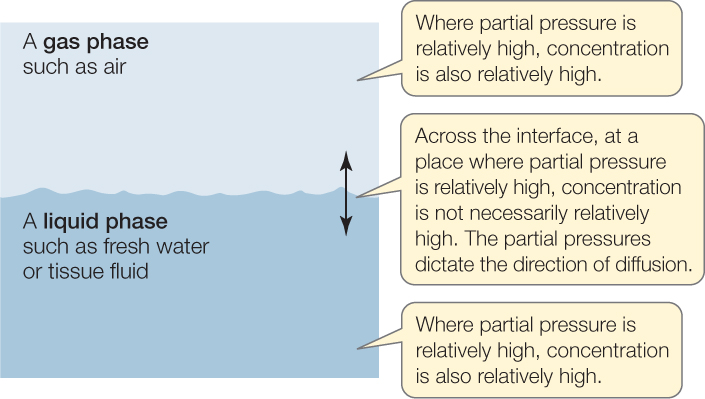
Figure 31.2: A Gas Always Diffuses from High Partial Pressure to Low Partial Pressure The concepts illustrated here apply to any gas. The gas is assumed to be in solution in the liquid phase. Within a gas phase or within a liquid phase, the partial pressure and concentration of a gas are proportional. Thus as the gas diffuses from high to low partial pressure, it also diffuses from high to low concentration. Concentration, however, is not a guide to diffusion across an interface between a gas and a liquid phase. Diffusion occurs from high to low partial pressure, but this does not necessarily mean the gas moves from where its concentration is high to where its concentration is low.